Abstract
At present, the mainstream industrial synthesis method of polyamide is still bulk fusion polymerization, which is not in line with the concept of green synthesis because of the high threshold of reaction conditions and large energy consumption. Based on this, a new synthesis route of polyamide elastomer was studied in this paper. By synthesizing the end-functionalized long carbon chain diacrylamide (DDA) containing amide bonds, the synthesis mechanism was made by the thiol double bond Michael addition reaction. A series of polyamide elastomers with different ratios of hard and soft segments were synthesized by using dimercaptan chain extenders with different molecular weights. The results showed that the new synthesis route successfully prepared DDA and polyamide elastomers. The mechanical properties of the products were excellent, and the strain rate could reach 383 %. Tensile strength up to 23.08 MPa. The synthesis route has the characteristics of simple reaction process, mild reaction conditions and high reaction conversion rate. It provides theoretical guidance for the new synthesis route of polyamide elastomer.
1. Introduction
Elastomers such as polyester and polyurethane, which started early in development, have advantages such as light weight, wear resistance and good elastic recovery, but their performance is often unsatisfactory in the face of higher mechanical properties [1-3]. In contrast, polyamide elastomers have attracted the attention of researchers due to their unique molecular structure and many excellent properties [4-5]. The combination of the flexibility provided by the soft segment of the molecular chain and the unique physical cross-linking effect of the amide bond gives the polyamide elastomer excellent mechanical strength and good chemical weather resistance, wear resistance and corrosion resistance [6].
At present, the main industrial synthesis method of polyamide is still bulk fusion polymerization. The process of this method is mature and the product performance is stable, but the threshold of reaction conditions is high, the requirements of temperature control and regulation are stricter, which is not in line with the concept of green synthesis. The idea of He Jie et al. [7] to synthesize polyamide through Thiol-Ene click reaction was impressive. However, the disadvantages of low industrial activity of functional precursor synthesis of this method still need to be solved.
In this paper, the end-functionalized long carbon chain diacrylamide precursor containing amide bond was synthesized by substituting didodecylamine with acrylyl chloride. The chain extension was achieved by the Michael addition reaction of dimercaptan and the synthetic precursor double bond, and the molecular chain structure of polymer was constructed and the molecular weight was improved. Thanks to the characteristics of low energy consumption, fast reaction speed and high conversion rate [8], the new synthesis route has the advantages of low energy input and low mass loss, which provides a certain theoretical guidance and application basis for the new energy-saving and greening industrial production route of polyamide elastomers.
2. Experimental part
2.1. Experimental raw materials
The main experimental raw materials are shown in Table 1.
Table 1Main experimental materials and reagents
Name | Specification | Manufacturer |
1, 12-diaminododecane (DDDA) | 97 % | Shanghai Adamas Reagent Co., Ltd |
N, N-dimethylformamide | AR | Shanghai Macklin Biochemical Technology Co., Ltd |
1, 4-butyl dimercaptan | 99 % | Shanghai Bide Pharmatech Technology Co., Ltd |
1, 6-hexa dimercaptan | 99 % | Shanghai Bide Pharmatech Technology Co., Ltd |
1, 8-octyl dimercaptan | 99 % | Shanghai Bide Pharmatech Technology Co., Ltd |
1, 10-decamethylene mercaptan | 99 % | Shanghai Bide Pharmatech Technology Co., Ltd |
2.2. Experimental instruments
Experimental instruments are shown in Table 2.
Table 2Instruments used in the experiment
Instrument name | Model number | Manufacturer |
Electronic analytical balance | CP214 | Ohaus Instrument (Changzhou) Co., Ltd |
Intelligent magnetic stirrer | ZNCL-G130*70 | Gongyi Yuhua instrument Co., Ltd |
Electric thermostatic air drying oven | DHG-9076A | Shanghai Jinghong Experimental Equipment Co., Ltd |
Rotary evaporator | RV 3 ECO S096 | IKA(Guangzhou) Instrument Equipment Co., Ltd |
Electronic universal material testing machine | Instron 5943 | Illinois Tool Works Company |
Ultrasonic cleaning machine | PL-S30 | Dongguan Kangshijie Ultrasonic Technology Co., Ltd |
2.3. Preparation of polyamide elastomer
The “12” in PAE-12+X represents the carbon number of amines in the synthesis of bisacrylamide prepolymers, and the “X” represents the carbon number of mercaptan used in the synthesis of elastomers, such as: PAE-12+4 represents the polyamide elastomer synthesized from 1, 12-diaminododecane and 1, 4-butanedimercaptan.
2.3.1. Synthesis of dodecane bisacrylamide prepolymers (DAA)
DAA are prepared by polymerization of 1, 12-diaminododecane (DDDA) with Acryloyl Chloride. First, 5.01 g (0.025 mol) DDDA was taken into a 250 mL round-bottom four-mouth flask, about 100 mL Acetone was added as the solvent, and the DDDA was dissolved by ultrasound at 50 ℃ until the solution was clear and transparent. 14.87 g (0.075 mol, 1.5 equivalent) Triethylamine was added into the container as the acid-binding agent of the reaction, and magnetic stirrer was added at the same time, stirring under the condition of ice bath for 1 hour; The analytical balance was used to accurately weigh 4.53 g (0.05 mol) of acrylyl chloride and transfer it to the constant pressure drop funnel. At the same time, 15 mL of acetone solvent was added to dilute acrylyl chloride to prevent the reaction from being too violent. After vacuum treatment, argon gas was injected into the reaction system, and the acryloyl chloride solution in the droplet funnel was slowly added to the four-mouth flask within 1 hour under the atmosphere of argon. After the droplet was finished, the ice bath was removed and the reaction continued at room temperature for 12 hours. Finally, the crude product was separated by column chromatography, the product was confirmed and collected by nuclear magnetism, and the pure DAA was obtained and dried for use.
2.3.2. Synthesis of polyamide elastomers
Table 3Feed ratio of TAE series polyamide elastomer synthetic raw materials
Sample | DAA (g) | Mercaptan (g) | DBN (g) |
PAE-12+4 | 6.17 | 2.45 | 0.15 |
PAE-12+6 | 6.17 | 3.01 | |
PAE-12+8 | 6.17 | 3.57 | |
PAE-12+10 | 6.17 | 4.13 |
The feed is shown in Table 3. Take PAE-12+10 as an example: The prepared 6.17 g (0.02 mol) of dodecane diacrylamide prepolymer was added to 250 mL of four-mouth round bottom flask, and 50 mL of N, n-dimethylformamide (DMF) after re-steaming and removing water was added to dissolve the raw material, the system was placed in a protective atmosphere and stirred, and the reaction system was heated in an oil bath. Set the oil bath temperature at 60 ℃; Weighing 4.13 g (0.02 mol) 1, 10-decanedithiol and 0.15 g (3% of functional group content) catalyst 1, 5-diazabicyclic [4.3.0] nona-5-ene (DBN) and using a constant pressure droplet funnel to control the droplet acceleration rate and ensure that the droplet is finished within 30 minutes. After 12 hours of reaction, the reaction solution was separated by coprecipitation method, and the obtained product was poured into the polytetrafluoroethylene mold and placed in the vacuum oven at 80 ℃ to dry into film.
2.4. Characterization and performance test of PAE series polyamide elastomers
The characterization performance test of PAE series polyamide elastomers includes Nuclear magnetic resonance hydrogen spectroscopy characterization, Fourier infrared spectroscopy characterization and Uniaxial tensile test.
Nuclear magnetic resonance hydrogen spectroscopy (1H NMR) was tested using Avance 400 MHz nuclear magnetic resonance instrument manufactured by Bruker, Germany. About 5 mg of the sample was weighed by an analytical balance and dissolved in 0.50 mL deuterated N, N-dimethylformamide reagent (DMF-d7), and tetramethylsilane (TMS) was selected as the internal standard. The NMR hydrogen spectrum of the product was obtained by scanning at 80 ℃.
The PAE series elastomer selects the Alpha II Fourier transform infrared spectrometer produced by Bruker, Germany. The resolution is set to 4 cm-1, the number of scans of the sample is set to 32, and the scanning range of the spectrum is between 4000 cm-1 and 500 cm-1.
The uniaxial tensile test of PAE material is carried out on the TH-8203A tensile test machine produced by Suzhou Tuobo Machinery Equipment Co., LTD. The reference standard was GB/T 528-2009, the test condition was room temperature (23±2 ℃, 50±10%RH), and the constant loading rate was set at 100 mm/min.
2.5. Results and discussion
2.5.1. Structural design and characterization
This synthesis route mainly uses long carbon chain amines with double-terminated amino groups. As shown in Fig. 1, 12-diaminododecane and acrylyl chloride are substituted to synthesize a double acrylamide monomer with an amide bond, namely DAA. DAA and dimercaptan with different carbon numbers are subjected to Michael addition reaction to expand the chain, and polyamide elastomer is finally synthesized with the increase of reaction degree. As Michael addition reaction is a click reaction, it has the advantages of fast speed, high conversion rate and mild reaction conditions, and the reaction process can be carried out in DMF solvent at 60 ℃, greatly simplifying the reaction process.
Fig. 1Synthesis route of PAE-12+X series polyamide elastomer
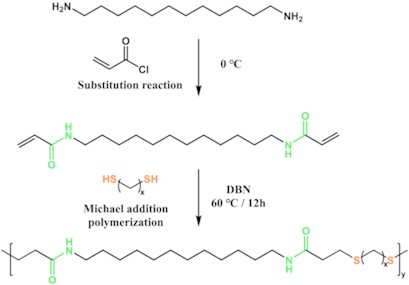
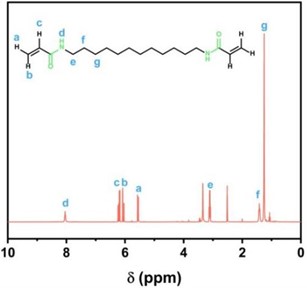
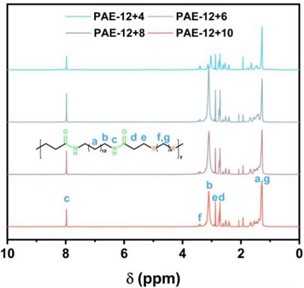
Fig. 2a) 1H NMR diagram of DAA monomer; b) 1H NMR diagram of PAE-12+X series polyamide elastomer
a)
b)
Fig. 2(a) shows that with the solvent peak of deuterated DMSO at 2.52ppm and deuterated water at 3.35 ppm. a, b and c are the absorption peaks of proton-hydrogen on the carbon-carbon double bond, and the positions are 6.06, 5.56 and 6.21 ppm, respectively. d is the position of the absorption peak of the proton hydrogen of the amino group on the amide bond, which is 8.04 ppm; The absorption peak at 3.11 ppm is attributed to proton-hydrogen e on the secondary carbon atom connected to the amide bond, and the peak of proton-hydrogen f on the secondary carbon atom connected to the secondary carbon atom is 1.42 ppm. The peak of proton-hydrogen g absorption on the remaining secondary carbon atoms is about 1.25 ppm. The results of 1H NMR analysis of the synthesized monomer showed that DAA monomer was successfully synthesized.
Fig. 2(b) shows that a is the proton hydrogen absorption peak of ten secondary carbon atoms in 1, 12-diaminododecane away from the amino group, and the position is about 1.2-1.8 ppm. b is the proton hydrogen absorption peak of the secondary carbon atom connected to the secondary amine group side of the amide bond, and the position is 3.10 ppm.c is the absorption peak position of protone-hydrogen of amide bond, which is 7.97 ppm. d is the proton hydrogen absorption peak of the secondary carbon atom connected to the carbonyl side of the amide bond, located at 2.74 ppm; At 2.88 ppm, the absorption peak is attributed to the proton hydrogen e on the secondary carbon atom connected to the sulfur atom and the secondary carbon atom to which d belongs. On the other side of the secondary carbon atom connected to the sulfur atom, the absorption peak of proton-hydrogen f is 3.41 ppm. The peak of proton hydrogen absorption of the secondary carbon atoms of the other mercaptan carbon chains is located at g, and the peak location is between 1.2 and 1.8 ppm. The results of 1H NMR analysis showed that PAE-12+X series polyamide elastomers were successfully synthesized.
2.5.2. Spectral analysis
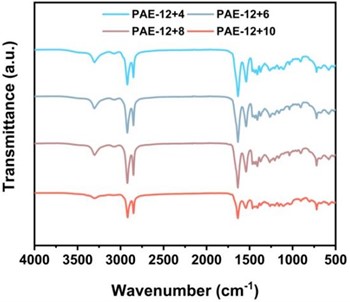
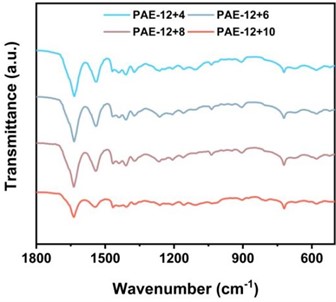
As shown in Fig. 3(a), the absorption peak around 3301 cm-1 came from the N-H stretching vibration of hydrogen bond association in the amide bond. As shown in Fig. 3(b), in the fingerprint region, the C = O stretching vibration peak of the amide I band appears around 1638 cm-1, which is composed of hydrogen bond association and the free C = O stretching vibration peak. The amide II band appears around 1546 cm-1 and is considered to be composed of C-N stretching vibration peaks associated with hydrogen bonds and N-H in-plane bending vibration peaks. The -CH-bending vibration peak in the aliphatic carbon chain is at 1468 cm-1. The absorption peaks at 1261 and 1210 cm-1 are generally considered to be caused by the coupling of the free and hydrogen bond associated C-N stretching vibration in the amide III band with the in-plane bending vibration of N-H.
Fig. 3PAE-12+X series elastomers in a) 500-4000 cm-1; b) Infrared absorption map at 500-1800 cm-1
a)
b)
2.5.3. Mechanical properties analysis
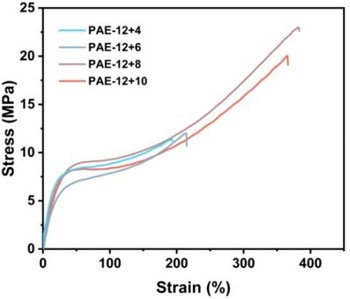
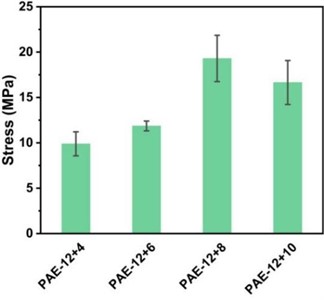
As shown in Fig. 4, the test results show that the sample with the best comprehensive mechanical properties is PAE-12+8, whose strain rate is about 383 % and tensile strength is up to 23.08 MPa. From PAE-12+4 to PAE-12+8, the elongation at break and tensile strength of the samples increased, but from PAE-12+8 to PAE-12+10, the elongation at break and tensile strength decreased slightly. This is caused by two factors. Due to the entanglement and curling of the molecular chain in the soft segment of polyamide elastic molecular chain under the physical cross-linking of the hard segment, the physical cross-linking network gradually deforms and fails during the stretching process, and the tangled soft segment will further extend, so the sample with more carbon number in the soft segment will have greater elongation at break. In the face of high-speed impact tensile, the hydrogen bonds between the molecular chains of polyamide elastomers with appropriate ratio of soft and hard segments can be more fully depolymerized to cope with the impact, which can effectively prevent the slip of molecular chains and enhance its mechanical strength, so the tensile strength and elongation at break of PAE-12+4 to PAE-12+8 are improved. To sum up, the mechanical properties of PAE elastomers can be optimized by controlling the proportion of soft and hard segments in the molecular structure design.
Fig. 4a) Uniaxial tensile stress-strain curve of PAE-12+X series elastomers; b) comparison of uniaxial tensile strength and error bar
a)
b)
3. Conclusions
In this paper, a double acrylamide monomer (DAA) with amide bond was synthesized by substituting long carbon chain amines with acrylyl chloride. A series of polyamide elastomers with excellent mechanical properties were synthesized by using 1, 4-butyldimercaptan,1, 6-hexyl dimercaptan,1, 8-octanedimercaptan and 1, 10-decanedimercaptan through the mercaptan - olefin Michael addition click reaction. The results of hydrogen NMR spectroscopy and infrared spectroscopy proved that the elasticity of polyamide was successfully prepared by a new synthesis route. The mechanical test results show that PAE-12+X series elastomer exhibits a relatively consistent stress-strain trend, among which the mechanical properties of PAE-12+8 are better, the strain rate is up to 383 %, and the tensile strength is up to 23.08 MPa. Compared with the traditional synthesis route of acid and amine condensation preparation of polyamide, the design idea of this synthesis route has the characteristics of simple reaction process, mild reaction conditions and high reaction conversion rate, which reduces the process cost to a certain extent, conforms to the concept of green chemistry, and provides a new idea for the synthesis of high-performance polyamide elastomers in the future.
References
-
S. R. Petersen et al., “Ultra‐tough elastomers from stereochemistry‐directed hydrogen bonding in isosorbide‐based polymers,” Angewandte Chemie International Edition, Vol. 61, No. 17, pp. 1–8, Apr. 2022, https://doi.org/10.1002/anie.202115904
-
S. Gong et al., “Thermoplastic polyamide elastomers: synthesis, structures/properties, and applications,” Macromolecular Materials and Engineering, Vol. 306, No. 12, pp. 1–23, Oct. 2021, https://doi.org/10.1002/mame.202100568
-
X. Qi, J. Zhang, L. Zhang, and D. Yue, “Bio-based self-healing Eucommia ulmoides ester elastomer with damping and oil resistance,” Journal of Materials Science, Vol. 55, No. 11, pp. 4940–4951, Jan. 2020, https://doi.org/10.1007/s10853-019-04272-3
-
Y. Huang, W. Li, and D. Yan, “Preparation and characterization of a series of polyamides with long alkylene segments: Nylons 12 20, 10 20,s 20,6 20, 4 20 and 2 20,” Polymer Bulletin, Vol. 49, No. 2-3, pp. 111–118, Nov. 2002, https://doi.org/10.1007/s00289-002-0090-3
-
C. Liu et al., “Polymers from fatty acids: poly (ω-hydroxyl tetradecanoic acid) synthesis and physico-mechanical studies,” Biomacromolecules, Vol. 12, No. 9, pp. 3291–3298, Sep. 2011, https://doi.org/10.1021/bm2007554
-
D. Quinzler and S. Mecking, “Linear semicrystalline polyesters from fatty acids by complete feedstock molecule utilization,” Angewandte Chemie International Edition, Vol. 49, No. 25, pp. 4306–4308, Jun. 2010, https://doi.org/10.1002/anie.201001510
-
H. Li, S. Thanneeru, L. Jin, C. J. Guild, and J. He, “Multiblock thermoplastic elastomersviaone-pot thiol-ene reaction,” Polymer Chemistry, Vol. 7, No. 29, pp. 4824–4832, Jul. 2016, https://doi.org/10.1039/c6py00822d
-
S. X. Li et al., “Preparation and Properties of high heat-resistant polyamide 62/polyethylene glycol diamine copolymers,” (in Chinese), Development and Application of Materials, Vol. 34, No. 1, pp. 80–87, 2019, https://doi.org/10.19515/j.cnki.1003-1545.2019.01.016
About this article
The authors have not disclosed any funding.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declare that they have no conflict of interest.

