Abstract
Objective: to provide definite science bases for its accurate identification and quality control. Methods: comparative identification on the stem, petiole and leaf of the two original plants of Clematidis armandii Caulis was carried out by the classical paraffin technique. Explore the index component for identification of Clematidis armandii Caulis by TLC and establish the quantitative analysis method by HPLC. Results: the rattan stems of Clematidis armandii Caulis, originated from Clematis armandii Franch and Clematis montana Buch.-Ham., are similar. However, they can be differentiated from each other by the structure of vascular bundle. Moreover, the structure of petiole are entirely different. The characteristic spots of beta-sitosterol in Clematidis armandii Caulis from six different places were detected by TLC. HPLC is high accurate, good reproductive, the mass concentration of beta-sitosterol in Clematis armandii Franch. was 189.18-190.27 μg·g-1 and in Clematis montana Buch.-Ham. was 336.90-338.34 μg·g-1. Conclusion: the spots were apparent and effective separated, when we used trichloromethane-ethyl acetate (10:1) for development, 10 % vitriol ethanol to color, and detected under the UV light (365 nm). This could be the TLC identification condition of beta-sitosterol in Clematidis armandii Caulis. Calculated on dry product by HPLC, the beta-sitosterol in Clematidis armandii Caulis could not be less than 0.018 %.
1. Introduction
Clematidis armandii is the dried cane of the Clematis armandii Franch or Clematis montana Buch.-Ham. which are ranunculaceae plants. It has the effect diuresis, used for gonorrhea, edema, upset urine, mouth sores, amenorrhea less milk, damp heat arthralgia syndrome [1]. In the market, Caulis Clematidis armandii has mixed species, it belong to a variety of plants which were often used as a mixture of Clematidis armandii, and it is difficult to identify the similar varieties [2]. In this study, using paraffin section identifies organizational structure of stems, leaves, petioles in the Clematis armandii Franch and Clematis montana Buch.-Ham., and establishing the standard atlas. Using the method of TLC to search index component and establishing the TLC identification with the control herbs. Selecting by the method of HPLC for screening quantitative analysis of the content determination method etc. Finally, providing basis for the improvement of the quality standard for Clematis armandii to better control the quality of drugs.
2. Apparatus and materials
LC-10A high performance liquid chromatography (Shimadzu), LC-10A pump, DGμ-12A online degasser, SPD-10A detector, SIL-10A autosampler, CTO-10A column oven, CLASS-VP5. 03 liquid chromatography workstation.
TC-C18 chromatographic column (Agilent, 4.6 m×250 mm, 5 μm). KQ5200DE CNC ultrasonic cleaner; hundred thousandth electronic analytical balance (BS100S), OLYMPUS BX51 microscope system of digital integration (Image-Pro Plus 6.0 Chinese version control software).
Beta-sitosterol Reference substance (Chinese Academy of Food and Drug Testing, Lot: 110851-201, 206), methanol (chromatographically pure, American Tedia company), silica gel G (chemically pure, Qingdao waves silicone dryer plant). Herbs were collected from six different origins (see Table 1), which were identified by Song Liang Ke. In addition, the five batches of Clematidis armandii Caulis were purchased from Liangshan Yi Autonomous Prefecture, Sichuan Aba Tibetan and Qiang Autonomous Prefecture, Sichuan Beichuan County, Zhaotong in Yunnan and Sichuan thousand square Chinese Herbal Medicine Co, Ltd.
Table 1The message of Clematidis armandii Caulis
The samples | No. | Habitat | Collecting time |
Clematis armandii Franch | 1 | Gaoqiao, Emei, Sichuan | 2013-4-25 |
2 | Gaomiao, Hongya, Sichuan | 2013-4-20 | |
3 | Xiaxi, Mabian, Sichuan | 2013-5-9 | |
Clematis montana Buch.-Ham. | 4 | Leidongping, Emei, Sichuan | 2013-4-27 |
5 | Sanhekou, Mabian, Sichuan | 2013-5-30 | |
6 | Longchi, Emei, Sichuan | 2013-5-25 |
3. Methods and results
3.1. Microscopic identification
1) The structure of the stem.
Clematis armandii Franch: a list of pericytes, round, the outermost layer of the cortex is almost square, arranged closely, and have no cell gap. The inner parenchyma cells the cortex are irregular round, and have cell gap. There are two sclerenchyma rings in the phloem, and more from the outer layer of thick-walled cells 6-9, was not continuous cap structure; continuous arcuate inner mostly composed of 1-2 layers of thick-walled cells box. Xylem wide, large ducts, wood fibers, wood parenchyma cells were wood of rings is not obvious. Myeloid round, wood technology, there is the cell gap, sometimes by a thin wall of the central marrow of non-wood cells or hollow (Fig. 1).
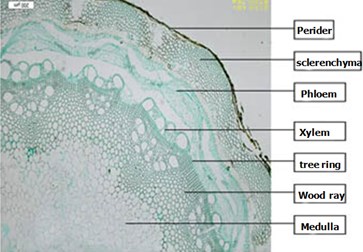
Clematis montana Buch.-Ham.: Hydrangea vine biennial stem visible by decadent tissue rhytidome. The cap structure of outer ring by the discontinuous 8-17 layers of thick wall cells, the inner ring is not obvious. Xylem large, scattered holes arrangement, ray cells lignified, rings clear. With the wooden through stems of distinction (Fig. 2).
Fig. 1Clematis armandii Franch Stems
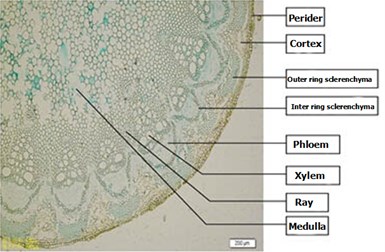
Fig. 2Clematis montana Buch.-Ham. stems
2) Leaf structure of the organization.
Clematis armandii Franch Stems’s leaf epidermal cells are square or rectangular category, closely arranged neatly outside the cuticle. Palisade tissue is typically 2 column cells, the spongy tissue of 5-6 rows of cells. Main vein vascular bundle surrounded by a thick fibrous oval, semicircular phloem, xylem and more from the catheter components.
Clematis montana Buch.-Ham. has mainly 4-5 rows of cells in the spongy tissue, cyclic structure with wooden main veins Vascular outside without a thick fibers pass to be different.
3) The organizational structure of the petiole.
Irregular cross-section through the petiole wooden round. Epidermal cells an outer cuticle. Outermost cortical cell types square, arranged very close, no cell gap; cortical parenchyma cells in the inner irregular round, there is the cell gap. Vascular bundles are mostly six, evenly distributed, thick-walled cells in the outer layer of 2-6, was cap. Phloem tissue mostly irregular round or square class. Xylem from the catheter, wood parenchyma cells and wood fibers, rays broad, often small vascular bundles visible secondary cell wall thicker, slightly wood. Pith cells round, the cell gap (Fig. 3).
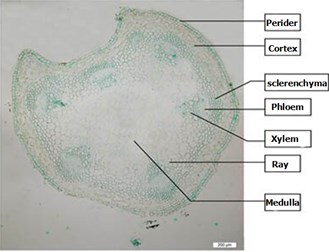
Clematis montana Buch.-Ham. adaxial petiole pale heart-shaped cross-section, of vascular 6, but both sides of the vascular bundles near the center of the shaft surface often see two small secondary vascular bundle, vascular bundle outside layer of thick-walled cells often 3-10 can pass the difference between wooden (Fig. 4).
Fig. 3Clematis armandii Franch Stems petiole
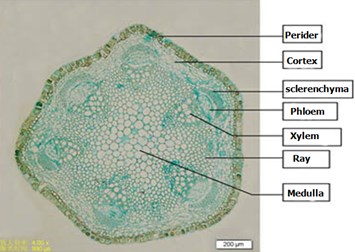
Fig. 4Clematis montana Buch.-Ham. petiole
3.2. TLC identification of the chemical composition
1) Preparation of the test solution and reference solution.
a) Preparation of the test solution;
2 g medicinal powder (over 4 screens), 100 ml alcohol, reflux for 2 hours, filtration, the filtrate evaporated, the residue was dissolved with 5 ml ethanol, static, the supernatant as the standard medicine solution.
b) Preparation of reference solution;
Beta-sitosterol, add anhydrous ethanol, as the reference solution (1 mg/ml).
2) TLC conditions and results.
According to Thin-layer chromatography (Chinese pharmacopoeia 2010 edition), draw the test solution of 8 μ L, 4 μ l reference solution, respectively, on the same silica gel G thin layer plate. Developer: 1) chloroform:acetone (25:1); 2) cyclohexane:ether:acetate (20:5.5:2.5); 3) toluene:chloroform:acetone:methanol (8:5:1:1); 4) chloroform:ethyl acetate (10:1); 5) petroleum ether:ethyl formate:formic acid (6:2:0.1), spray with 10 % sulfuric acid ethanol solution, the color clear heating at 105 ℃ to spots, respectively the sunlight and UV lamp (365 nm).
Chloroform-ethyl acetate (10:1) used as a Developer will have good separation effect (Fig. 5).
3.3. Determination of beta-sitosterol
1) Preparation of the solution.
a) Preparation of the test solution;
1 g medicine powder (over 3 screens), accurately weighed, were set stoppered Erlenmeyer flask, precision chloroform 50 ml, sonication (power 120 W , frequency 40 kHz) 2 hours, cooled, shake, filtration, the filtrate evaporated and the residue dissolved in methanol, set 5 ml flask, microporous membrane (0.45 μm) filter.
b) Preparation of standard solution;
Beta-sitosterol accurately weighed, add methanol, as the standard solution (65 μg/ml).
Fig. 5Clematidis armandii Herbs TLC: 1-3: Clematis armandii Franch; 4-6: Clematis armandii Franch; 7: Liangshan prefecture Sichuan; 8: Anba prefecture Sichuan; 9: Beichuan County Sichuan; 10: Zhaotong Yunnan; 11: Slices; 12: Beta-sitosterol
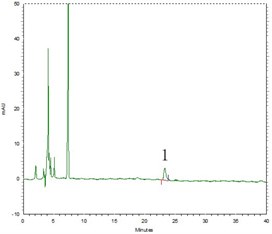
Fig. 6High performance liquid chromatography (HPLC) figure (1 – Beta-sitosterol)
a) Reference substance
b) Blank solvent
c) Clematis armandii Franch
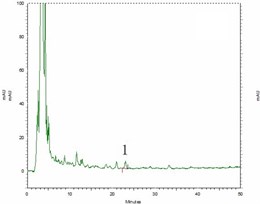
d) Clematis armandii Franch
2) Chromatographic conditions.
TC-C18 column (Agilent, 4.6 mm×250 mm, 5 μm); mobile phase:methanol-water (97:3); detection wavelength: 204 nm; column temperature: 30°C; flow rate: 1.0 ml/ min; injection volume: 10 μL. Number of theoretical plates beta-sitosterol of not less than 3000.
3) Methodological study.
a) Methods specificity study;
Respectively pipette beta-sitosterol standard solution, blank solution, test solution (Clematis armandii Franch, Clematis montana Buch.-Ham.) each 10 μl, injection, according to 2.3.2 test, both beta-sitosterol standard solution and test solution appear beta-sitosterol absorption peaks at about 23 min (Fig. 6).
b) Linear relation;
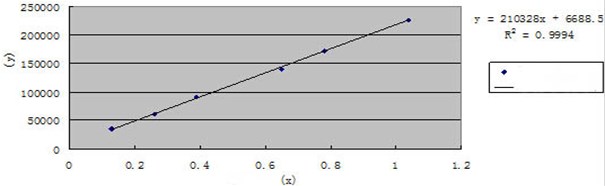
Take beta-sitosterol standard solution (65 μg /ml), respectively, sample 2 μl, 4 μl, 6 μl, 10 μl, 12 μl, 16 μl, with the sample volume (X, μg) for horizontal , peak area values (Y) for the vertical axis, the establishment of a standard curve, the linear regression equation Y= 210328X+6688.5, (r= 0.9997), the linear range of 0.13-1.04 μg (Fig. 7).
Fig. 7The standard curve of Beta-sitosterol
c) Precision test;
At a concentration of 65 μg/ml of the standard solution, continuous injection six times, measured the peak area, RSD is 1.67 %, indicating that equipment’s precision is good.
d) Repeatability;
Parallel to take No. 1 and No. 4 Sample 6 copies, accurately weighed, according to the sample preparation method, measured the peak area by sample analysis, the No. 1 sample’s peak area RSD is 2.31 % and the No. 4 sample’s peak area RSD is 2.11 %, indicating that the reproducibility of the test method is well.
e) Stability test;
Take the same test solution, according to the above chromatographic conditions measured peak area after the preparation of 0 h, 2 h, 4 h, 6 h, 8 h, 12 h, 24 h, Clematis armandii Franch and Clematis montana Buch.-Ham. peak area RSD values were 2.19 %, 2.94 %, indicating that the test solution was stable within 24 h.
Table 2Recovery of Clematis armandii Franch
Sample (g) | Beta-sitosterol (μg) | Standard solution (μg) | Total measure (μg) | Recovery (%) | Average (%) | RSD (%) |
0.5003 | 85.18 | 85 | 171.4779 | 101.5269 | 101.09 | 1.62 |
0.5169 | 88.01 | 85 | 175.6762 | 103.1367 | ||
0.5402 | 91.98 | 85 | 176.3299 | 99.23518 | ||
0.5205 | 88.62 | 85 | 175.6405 | 102.3771 | ||
0.5291 | 90.09 | 85 | 176.0565 | 101.1371 | ||
0.5058 | 86.12 | 85 | 170.3605 | 99.10647 |
Table 3Recovery of Clematis montana Buch.-Ham.
Sample (g) | Beta-sitosterol (μg) | Sandard solution (μg) | Total measure (μg) | Recovery (%) | Average (%) | RSD (%) |
0.5003 | 152.34 | 150 | 304.0098 | 101.1132 | 100.40 | 0.74 |
0.5049 | 153.74 | 150 | 305.2176 | 100.9851 | ||
0.5102 | 155.36 | 150 | 306.1638 | 100.5359 | ||
0.5317 | 161.91 | 150 | 312.2349 | 100.2166 | ||
0.524 | 159.56 | 150 | 308.1325 | 99.04833 | ||
0.5309 | 161.66 | 150 | 312.4169 | 100.5046 |
f) Recovery test;
Accurately weigh sample No. 1 and No. 4 each 0.5 g (1 g Clematis armandii Franch contains beta-sitosterol 170.266 μg, 1 g Clematis montana Buch.-Ham contains beta-sitosterol 304.505 μg), each 6 parts, precision adding beta-sitosterol standard solution, according to the sample preparation method, and measuring beta-sitosterol content, calculate recoveries, the results in Table 2 and Table 3.
4) The content determination of the sample.
Take samples collected from six different regions and buy medicinal materials from five different places, according to the sample preparation method, preparation of sample solution, determine the content of beta-sitosterol, the results are shown in Table 4.
Table 4Results
Sample | No. | Beta-sitosterol content (μg·g-1) (weigh dry) |
Clematis armandii Franch | 1 | 189.18444 |
2 | 190.2611 | |
3 | 189.5256 | |
Clematis montana Buch.-Ham | 4 | 338.3389 |
5 | 336.9067 | |
6 | 337.4978 | |
Liangshan Yi autonomous prefecture | 7 | 156.945 |
Sichuan Aba Tibetan and Qiang Autonomous prefecture | 8 | 241.6545 |
Sichuan Beichuan county | 9 | 168.716 |
Zhaotong in Yunnan | 10 | 176.599 |
Sichuan thousand square Chinese Herbal Medicine Co., Ltd. | 11 | 153.8656 |
4. Discussion
Chinese pharmacopoeia 2010 edition provisions of oleanolic acid as reference [3]. In this study, samples were processed by the Chinese pharmacopoeia method, using five different deployment system [4-8], TLC results were not displayed oleanolic acid, but showed a clear beta-sitosterol spots. Beta-sitosterol has anti-inflammatory, antipyretic effect, the effects are significant, and for the treatment of periodontitis all disease and oral ulcers [9, 10], which is consistent with some part of the effect of Clematidis armandii Caulis. Although commercially available Clematidis armandii Caulis contain beta-sitosterol, but in no better condition index components can be beta-sitosterol as an indicator. HPLC method for the determination of this study established high precision, repeatability, Clematidis armandii Caulis content in beta-sitosterol is not less than 0.017 %. May be one of the bases as Clematidis armandii Caulis quality standards established.
References
-
Chinese Pharmacopoeia. Vol. 34, 2010.
-
Pei Jin, Wan De-guang, Cai Wenlong, et al. Chuanmutong quality control. Eighth National Conference of Medicinal Plants and Herbal Medicines, Hohhot, 2009.
-
Chinese Pharmacopoeia. Vol. 25, 2010.
-
Yan Hua, Wang Pao Chin, Lu Jing Thin-layer chromatography separation of oleanolic acid and ursolic acid. Journal of Pharmaceutical Analysis, Vol. 29, Issue 12, 2009, p. 2168-2170.
-
Zhang Jianping, Hai Hua, Liu Haifeng, et al. Duchesnea identification of oleanolic acid. Mudanjiang Medical College, Vol. 31, Issue 1, 2010, p. 57-58.
-
Lu Xiang E., Huang Zhi Loquat and oleanolic acid determination. Huaxi Pharmaceutical Journal, Vol. 24, Issue 5, 2009, p. 561-562.
-
Li Jin, Li Yang Traditional and herbs and other quality standards Aralia study. Journal of Medical Research, Vol. 39, Issue 12, 2010, p. 82-84.
-
Chao Chi Mao, Zhang Nan, Tang Chunfeng, et al. 36 kinds of oily medicine identification unsaponifiables TLC. Chinese Journal of Experimental Traditional, Vol. 18, Issue 3, 2012, p. 79-83.
-
Han Junhua Nature of phytosterols, function and application. International Journal of Health Toxicology, Vol. 28, Issue 5, 2001, p. 285-291.
-
Zuo Yu Phytosterols research and application. Food and Grease, Vol. 7, 2012, p. 1-4.
