Abstract
While the world population is increasing day by day, environmental problems are reaching a level that cannot be ignored. Environmentalist steps are being taken in many areas, and governments are resorting to sanctions. It aims to reduce fossil fuel use as an environmental step in the transportation industry. Increasing the use of electric vehicles will be significant progress in achieving this goal. Batteries are generally used to store energy in electric vehicles. However, besides the weight problem and insufficient power density of the batteries, they have disadvantages, such as being produced from environmentally harmful materials. In this context, new energy storage technologies are being researched. One of them is “supercapacitor” technology. This paper is a review article examining several aspects of supercapacitors.
Highlights
- Emphasis in this paper is to examine capacitor, supercapacitor technologies.
- Explanation of types of capacitors and supercapacitors.
- Explanation of types of electrodes, electrolytes and separators used in supercapacitors.
- Evaluation of supercapacitor structure.
- Evaluation of supercapacitor technology.
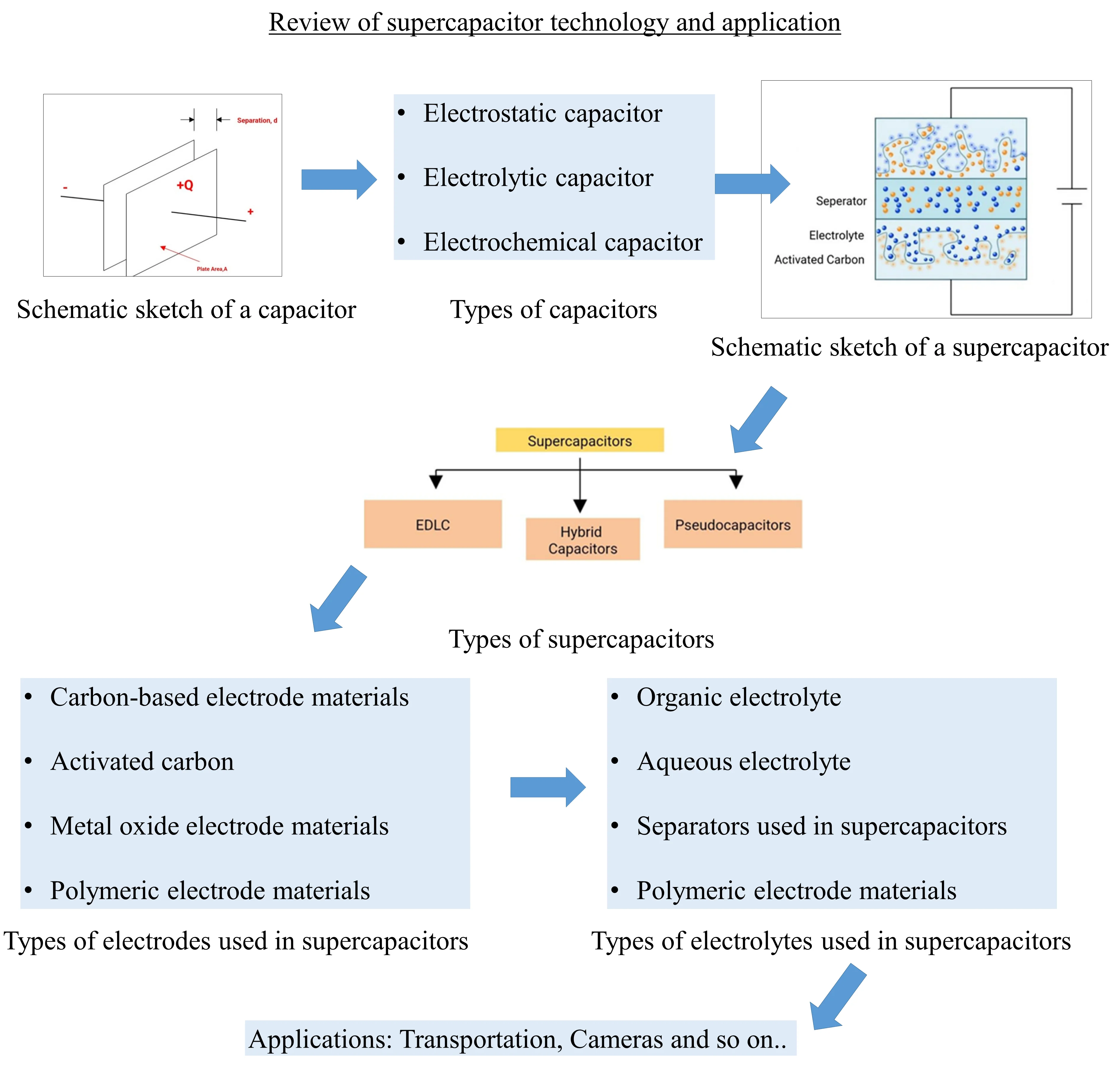
1. Introduction
Electric vehicles are an essential environmental step today. All-electric vehicles are called “zero-emission vehicles” because no harmful gases are released [1]. Gas emissions are a critical environmental problem. Due to the greenhouse effect created by the increasing CO2 emissions in the atmosphere, serious changes have occurred in the earth’s surface temperature and climate. Moreover, according to 2002 data, it is estimated that 40,000 deaths per year are due to air pollution in Austria, France, and Switzerland combined (approximately 74.5 million population). These estimates are based on the assumption of linearity of exposure-response relationships [2]. CO2 emissions from the transportation industry can be reduced by electric vehicles. In 2007, it was proposed by the European Union to reduce greenhouse gas emissions by 20 % by 2020 compared to 1990 levels. Goals like these are gaining importance every day. Environmental problems in the world are now one of the main issues that need to be dealt with. Considering that the transport industry accounts for approximately 31 % of European energy use and about 25 % of European CO2 emissions (according to 2010 data), the importance of switching from conventional vehicles to electric vehicles can be better understood [3-4].
In all-electric vehicles, the main power source is usually a battery. A secondary battery or supercapacitor can be used as an additional source supporting the main battery. This paper discusses supercapacitors, a new version of the conventional capacitor, in detail. This paper explains the supercapacitor structure, types, classifications, and application areas. Although all application areas of supercapacitors are briefly mentioned, their use in electric vehicles is emphasized.
2. Capacitor
A capacitor is a passive electronic component consisting of a pair of conductive plates separated by a dielectric material. Figure 1 shows the schematic sketch of a capacitor. Capacitors store electrical energy as an electrostatic charge. Equal amounts of positive and negative charges are collected on opposite surfaces of the conductive plates, thus creating a voltage difference between these surfaces. When an external load connects these two surfaces, current flows through them until full charge balance is achieved, and then the stored energy is released. This is the charge-discharge cycle. If desired, it can be restored to the charged state by applying a voltage to the capacitor. No chemical reaction takes place during this process. The load is physically stored [5].
Fig. 1Schematic sketch of a capacitor [5]
![Schematic sketch of a capacitor [5]](https://static-01.extrica.com/articles/24103/24103-img1.jpg)
The capacitance value of a capacitor in farads (); is directly proportional to the surface area () of the electrodes and the dielectric constant () of the insulating separator. It is inversely proportional to the distance () between the electrodes. Capacitance is the ability of an object to store an electric charge. Equation 1 represents the capacitance value of a capacitor. The value of (coulomb) is the amount of charge stored in the capacitor, and (volt) is the potential difference between the plates:
When finding the capacitor's power, the capacitor’s internal resistances must be considered. The internal resistances of the capacitor are called ESR (Equivalent Series Resistance). Eq. (2) shows the calculation of the maximum power value () to be obtained from the capacitor, depending on the voltage () applied to the capacitor and the ESR value of the capacitor [6]:
2.1. Types of capacitors
Conventional capacitors have been available since 1745 [7]. Later, they were developed and diversified and turned into technologies such as supercapacitors, which have an essential role in many applications, including electric vehicles today. Capacitors are basically of 3 types. These are electrostatic capacitors, electrolytic capacitors, and electrochemical capacitors. Fig. 2 shows the constructions of these three types of capacitors.
2.1.1. Electrostatic capacitor
The electrostatic capacitor is the most basic capacitor structure formed by placing an insulator between two conductors. Electrons are separated into opposite poles on opposite plates and stored in the electric field. The charge occurs when a potential difference occurs between the plates. It is the simplest type of capacitor.
Fig. 2Electrostatic, electrolytic, and electrochemical capacitors respectively [6]
![Electrostatic, electrolytic, and electrochemical capacitors respectively [6]](https://static-01.extrica.com/articles/24103/24103-img2.jpg)
2.1.2. Electrolytic capacitor
The structure of electrolytic capacitors is also similar to electrostatic capacitors. The only difference is that the electrodes are in direct contact with the electrolyte salt in electrostatic capacitors. Electrolytic capacitors are formed using a thin, structured dielectric material with a high dielectric constant on a rough metal surface. Although the energy densities of electrolytic capacitors are much higher than electrostatic capacitors, they have a shorter lifetime. They are much more likely to generate leakage current than an electrostatic capacitor.
2.1.3. Electrochemical capacitor
Electrochemical capacitors, also known as supercapacitors, have a much higher electrode surface area than other capacitor types. This way, it has much higher capacitance values than other capacitors. Although the working principles of conventional capacitors and supercapacitors are similar, energy storage systems differ [6]. In this study, supercapacitors are discussed in detail.
3. Supercapacitors
Supercapacitors are improved versions of conventional capacitors. Supercapacitors store electrical charge in a double layer at the interface between high surface area electrodes and electrode material. For this reason, it is also called a “double-layer capacitor”. Otherwise, they are also known by names such as ultracapacitor, electrical double-layer capacitor (EDLC), electrochemical capacitor (EC), power capacitor, gold capacitor, pseudo capacitor, and power caches. Different manufacturers have given these names, and there are slight differences between them. The evolution of supercapacitors is as follows; German physicist Hermann von Helmholtz first described the concept of a double-layer capacitor in 1853. The General Electric Company created the electrochemical capacitor operating on the basis of double-layer capacitance in 1957, but the device did not have any commercial use. Pinnacle Research Institute (PRI) named the device it produced an ultracapacitor. A double-layer capacitor was produced by the Standard Oil Company of Sohio (SOHIO) in 1966 in the format still used today. In this case, a group of Standard Oil engineers working on fuel cells accidentally discovered and patented the double-layer capacitor structure. However, supercapacitors were not used commercially for a long time. The first commercial device developed by the Japanese company Nippon Electric Company (NEC) in 1979 was called the supercapacitor, and it was the first commercially successful double-layer capacitor to come to market. After the 1980s, a number of companies were now producing supercapacitors [8-13].
3.1. Types of supercapacitors
According to the energy storage mechanism, supercapacitors are basically divided into three classes. These are electrical double-layer capacitors (EDLC), pseudo-capacitors, and hybrid capacitors. The classification of supercapacitors is shown in Fig. 3. Each class has a different charge storage mechanism. These mechanisms are non-faradaic, faradaic, and a combination thereof, respectively. Faradaic processes involve chemical reactions such as oxidation and reduction, and in these processes, charge transfer occurs between the electrode and the electrolyte. A non-faradaic process does not involve a chemical reaction. In this process, the charges are physically distributed on the surfaces [5, 14-16].
Fig. 3Types of supercapacitors
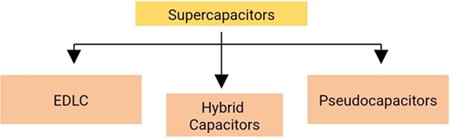
3.1.1. Electrical double layer capacitors (EDLC)
The charge accumulation mechanism of this structure is based on the principle of electrostatic separation of opposite charges in the double layer. In an ideal EDLC, only charge separation occurs at the electrode. No oxidation or reduction reactions take place. The process is not faradaic. Capacitance is independent of voltage. EDLCs store electrical charge directly across the bilayer of the electrode. No load transfer occurs across the interface. For this reason, a real capacitance effect is created. This structure is the basic supercapacitor structure [5, 10, 17].
3.1.2. Pseudo capacitor
Pseudo-capacitive supercapacitors involve the charge storage process by charging the double-layer layer at the electrode-electrolyte interface by faradaic charge transfer reactions. In addition to the capacitance formed by the separation of charges in the double layer, the additional capacitance value formed by the oxidation and reduction of the ions on the electrode surface is called pseudo-false capacitance. In pseudo capacitors, charge is stored through electrosorption, oxidation-reduction reactions and intercollation mechanism. In this structure, redox reactions occur, and the charge transferred is dependent on the voltage. For this reason, it is called a pseudo-capacitor [5, 18]. In short, pseudo capacitors, unlike other supercapacitors, store energy by chemical mechanism, just like in batteries [19]. Faradaic processes in pseudo capacitors enable these capacitors to achieve higher specific capacitance and higher energy density than EDLCs [20].
3.1.3. Hybrid supercapacitors
This construction is a combination of faradaic and non-faradaic components. In this way, a supercapacitor structure is obtained, providing continuous energy and exhibiting high capacitance for pulse power [5]. Hybrid supercapacitors use a carbon and a li-ion electrode. In this way, the faradaic electrode with high specific capacitance provides higher energy density. The non-faradaic electrode provides higher power density. The advantages of both processes (faradaic and non-faradaic) combine [20].
There are 3 types of hybrid supercapacitors based on the electrode configuration used. The first of these is the composite structure. In the composite structure, carbon-based materials are combined with polymer conductive or metal oxide materials. It is called binary composite or triple composite depending on the number of materials used in the electrode. Another type is the asymmetric structure. In this structure, EDLC is combined with the so-called capacitor electrode. Faradaic and non-faradaic processes coexist. In this structure, carbon material is used as the negative electrode, and polymer or metal oxide material is used as the positive electrode. Finally, there is the battery type hybrid supercapacitor structure. This structure is a combination of a battery electrode and a supercapacitor electrode [21].
EDLC is the most cost-effective and widely used supercapacitor type today. When talking about the supercapacitor concept, the structure usually discussed is the EDLC structure, which creates a real capacitance effect.
4. An overview of the supercapacitor structure
A supercapacitor consists of two electrodes, an electrolyte material and a separator. Although similar in structure to batteries, supercapacitors differ from batteries by containing a separator membrane that electrically separates the two electrodes [11, 17, 22]. Fig. 4 shows the supercapacitor structure. There are electrodes with increased surface area in the structure of the supercapacitor. In this way, the capacity value has increased. The energy density of a supercapacitor is much higher than that of a conventional capacitor [23], and a supercapacitor fills the gap between conventional capacitors and batteries in terms of energy density [22]. Electrode material, electrolyte type and separating membrane properties are the factors that determine performance criteria such as capacitance, cycle life, energy, and power densities in a supercapacitor [7, 17].
Fig. 4Schematic sketch of a supercapacitor [7]
![Schematic sketch of a supercapacitor [7]](https://static-01.extrica.com/articles/24103/24103-img4.jpg)
When a voltage is applied between the two electrodes in the supercapacitor, the positive and negative ionic charges in the electrolyte accumulate as opposite charges on the surface of the two electrodes. This process is physical, and no chemical reaction occurs inside the supercapacitor (the fake capacitor is an exception). The main difference between the supercapacitor from other capacitors is the expanded surface area of the electrodes. In this way, supercapacitors have a very high capacitance value compared to other capacitors. While the capacitance values for electrostatic capacitors are generally in the micro and millifarad range, the capacitance values of supercapacitors rise to the kilofarad level [6].
Supercapacitors can be classified according to the electrode material used or the type of electrolyte.
4.1. Types of electrodes used in supercapacitors
The electrode has a vital role in the performance of the supercapacitor. Performance criteria such as specific capacitance, life, power density, and energy density depend on the electrode. Since material properties directly affect performance, many studies are being conducted on this subject. Three types of electrode materials are used in supercapacitors. These; carbon-based materials can be called metal oxide materials or polymeric materials [17, 22]. Carbon-based materials are generally used in double-layer capacitors, while metal oxides and conductive polymer materials are used in pseudo capacitors [24-25].
4.1.1. Carbon-based electrode materials
Appropriate electrode materials with excellent electrical conductivity, high specific capacitance (energy stored per unit mass/volume or area of active material), large rate capacity, high surface area, and specific pore size are essential for supercapacitors as well as battery systems [25]. By looking at some features of the electrodes, it can be understood whether they provide these or not. The larger the surface area of an electrode, the greater the specific capacitance it will provide because charges are stored in the electrode's surface area. Surface areas can be increased by nanostructuring electrode materials. The specific capacitance of the electrode, which has high electronic and ionic conductivity, is high. Electronic and ionic conductivity can be achieved by adjusting the pores to an optimum size. If the material has small pores, the specific capacitance and therefore the energy density will be higher. On the other hand, in small pores the equivalent series resistance is (ESR) increases, which reduces the power density. Therefore, the pore size of the electrode material needs to be adjusted according to the application requirements. Generally, carbonaceous materials as electrode material in supercapacitors; It is mainly used in activated carbon, carbon nanotubes (CNTs), carbon nanofibers, carbon-based textiles, and graphene. These materials have high electronic conductivity, good mechanical performance, low cost, accessibility, and large surface area. But their low specific capacitance limits the energy density of the supercapacitor. Surface modification methods such as surface activation and heteroatom doping are used to improve capacitance. Among carbon materials, carbon-based textile is rarely used because of their small surface area. Carbon nanotubes (CNTs) were seen as the ideal electrode material, but although this material has good conductivity, a special one-dimensional pore structure, and good electrochemical stability, its capacitance is quite low. To solve this problem, studies have been carried out to increase the surface area by activating CNTs. The structure generally used as the electrode material at present is the activated carbon structure [14, 24, 26-29].
4.1.2. Activated carbon
While producing carbon materials from raw materials, different carbon structures (biochar, coal, hydrocoal, etc.) are obtained according to the method used. One of them is activated carbon. Activated carbon has many pores in its structure. There are many studies showing that the surface area of activated carbon and therefore the number of pores increase. A study conducted in 2002 found that the surface area of activated carbon was 3 times that of normal carbon. In addition, the pore volume of activated carbon increased by 1.5 times compared to non-activated carbon. These conclusions were made as a result of examination of activated carbon nanotubes and normal carbon nanotubes with transmission electron microscopy (TEM), scanning electric microscopy (SEM) and X-ray diffraction (XRD). In these examinations, it was clearly seen that when carbon nanotubes were activated, the nanotubes deteriorated and became structurally more curved and branched [30]. The carbon activation process causes degradation on the outer surface of the carbon nanotubes. These deformities help in expanding the surface area and pore volume required for the supercapacitor electrode. In part “Supercapacitors”, it was mentioned that Standard Oil engineers accidentally discovered the supercapacitor while working on the fuel cell. The engineers coated two aluminum electrodes with a layer of carbon 100 micrometers thick. The carbon was first chemically etched, resulting in many pores running across the surface like a sponge. Thus, an inner surface area was created approximately 100,000 times larger than the outer surface area. This process is said to “activate” the carbon [7, 30].
Many different methods are used to activate carbon. In addition, many other raw materials are used to obtain carbon. One is carbon production based on biomass (obtained from natural sources). Biomass-based carbon production is generally notable for being inexpensive and environmentally friendly. Activated carbon materials obtained from biomass are seen as promising electrode materials due to their chemical stability, microstructure, good conductivity, and large power density. The use of coconut shells in commercial activated carbon production is quite common. Activated carbon can be produced from natural biomass materials such as banana peel, cotton stalk, sugar cane pulp, corn cob, human hair, date seed, cherry seed, leaves, peanut shell, mulberry trees, etc. [24, 31-33].
Electrode costs are reduced with activated carbon obtained from biomass. This is because binder and conductive additives are not needed. For example, the banana peel has a self-adhesive material, and no adhesive is needed when producing activated carbon from the banana peel. In addition, the production process proceeds quite simply. In addition, recycling waste biomass is of great importance for the sustainable development of society. The only problem is that the electrodes obtained from activated carbon are thick. There are some applications where electrodes need to be ultra-thin, and carbon electrodes may not be suitable for this [31, 33].
The properties of carbon electrodes depend on the selected raw material, and the applied preparation technique. Basically, three raw materials are used in carbon applications. These; are polymers, minerals, and biomass. Among these, biomass is frequently preferred as a raw material in research due to its availability, low cost, and, most importantly, environmental friendliness [31].
4.1.3. Metal oxide electrode materials
Metal oxides such as RuO2 (Ruthenium(IV) oxide) or IrO2 (Iridium(IV) oxide) were preferred as electrode material in supercapacitors used for space and military applications. Supercapacitors with high specific power and high specific capacitance properties can be obtained with these materials. However, the costs are pretty high. In metal oxide supercapacitors, approximately 90 % of the capacitor cost is based on the electrode cost. In addition, this electrode has the limitation that it can only be used with aqueous electrolytes. Due to its cost, it is not possible to use it in commercial supercapacitors [22].
4.1.4. Polymeric electrode materials
When the polymer is used as the electrode material, the debate is still whether the device is a supercapacitor or turns into a battery. This is because a chemical reaction occurs when polymer material is used inside the capacitor. Considering the properties of the polymer material, high power and energy density values can be obtained when this material is used. However, the polymer material is not mechanically durable. Deformations such as swelling and shrinkage can often be seen. This situation may cause malfunctions during application [22].
4.2. Types of electrolytes used in supercapacitors
The electrolyte used in the supercapacitor has great importance in determining the maximum operating voltage, lifetime, and reliability of supercapacitors [24]. An electrolyte is a material that provides pure ionic conductivity between the positive and negative electrodes of a cell. Electrolytes can be liquid or solid [23, 34]. Electrolytes used in supercapacitors are divided into two classes: organic electrolytes and aqueous electrolytes.
4.2.1. Organic electrolyte
Organic electrolytes are generally used in commercial devices. The advantage of the organic electrolyte is the higher voltage values that can be achieved. The cell voltage is around 2.3-2.7 V when activated carbon and organic electrolyte are used together, while the cell voltage is about 0.8-1.0 V when the aqueous electrolyte is used. The energy density is directly proportional to the square of the cell voltage. For this reason, the energy density is higher when organic electrolytes are used. However, the organic electrolyte's internal resistance (ESR) is high. As a result, they exhibit low power values. The drop in power can be compensated by the high energy voltage. Organic electrolytes have disadvantages such as liquid leakage, toxicity, increased flammability, and corrosiveness. In systems using organic electrolytes, it is necessary to apply an anti-corrosion coating to the electrodes in order not to wear their electrodes. These disadvantages limit their use [22, 24, 29].
4.2.2. Aqueous electrolyte
In aqueous electrolytes, the unit cell voltage is limited to 1V. This means a very low cell voltage compared to organic electrolyte. However, the conductivity of the aqueous electrolyte is higher, and the cost is lower than that of the organic electrode [22].
The charge storage ability of the supercapacitor depends on the accessibility of the ions to the porous surface area. Therefore, the ion size in the electrolyte and the pore size in the surface area of the electrodes must be optimal. For this reason, the choice of electrode and electrolyte material should be made together [9].
4.3. Separators used in supercapacitors
The separator is a physical barrier placed between the electrodes to prevent a short circuit inside the supercapacitor (if a short circuit occurs, all the energy inside the device is released as heat in itself) [35]. Although the separator physically prevents contact between the electrodes, it allows ion passage [9, 36-37]. Separating membrane materials can generally be grouped as cellulose, fiberglass, textile, and polymer-based. Appropriate membrane selection plays a key role in the electrode-electrolyte interaction. The selection of the disconnector also directly affects the supercapacitor performance [17].
Generally, polymer and paper separators are used with organic electrolytes, and ceramic or glass fiber separators are used with aqueous electrolytes. For the best supercapacitor performance, the separator should have high electrical resistance, high ionic conductivity, and low thickness [9].
5. Supercapacitor applications
A low ESR (Equivalent Series Resistance) value is required to store energy quickly. Supercapacitors are the devices with the lowest ESR value compared to other energy storage devices. Supercapacitors can be charged-discharged very quickly due to their exceptionally low internal resistance and the absence of any chemical reaction in their internal structures. So from a speed standpoint, the supercapacitor can be an ideal store. A lower ESR value also means less power loss. Power losses occur in devices with large ESR values when the output load situation changes. This deteriorates the quality of the power supply and the performance of the electronic circuit. This sometimes causes electronic components to 'stuck' out of the blue. This can be observed on laptops or mobile phones. A low ESR value reduces the likelihood of device “stuck” [36, 38]. This is one of the reasons why super capacitors are preferred. Fig. 5 shows the power loss in a supercapacitor and a battery when the current drawn (output load state) changes.
When looking at energy storage technologies, fuel cells are considered systems with high energy density, and super capacitors are considered systems with high power density. On the other hand, batteries have a higher power density than fuel cells and a higher energy density than supercapacitors. From this point of view, they have an average power and energy value [34]. Different systems have different performances between energy and power. Therefore, a single system cannot meet the demand of different applications. The demand for each application is different. By using these three technologies together, suitable systems can be obtained. In a study by Paladini et al., these three systems were used together. While the fuel cell is the main power source, the battery and supercapacitor are secondary storage devices. In this system, the fuel cell both powers the electric motor and charges the battery and supercapacitor with the stored energy. In this system, the battery is only activated when the fuel cell is insufficient in high-power situations. If the required power is at a level the fuel cell can supply, the system is operated only with the fuel cell. If the power demand increases, the battery, and supercapacitor come into play and close the gap. In this system, the supercapacitor captures most of the braking energy, significantly improving fuel economy [35]. The storage of some of the energy that occurs during braking is called regenerative braking [39]. Because supercapacitors work fast, they are much more effective than batteries in efficiently recovering the energy that occurs in the case of rapid braking in vehicles [37].
Fig. 5The power loss in a supercapacitor and a battery when the current drawn changes [38]
![The power loss in a supercapacitor and a battery when the current drawn changes [38]](https://static-01.extrica.com/articles/24103/24103-img5.jpg)
For this reason, supercapacitors can be used in buses providing urban transportation. Thanks to the supercapacitor in these vehicles, which make many stop-starts during the day, most of the energy lost can be recovered. Another application where supercapacitors are used is rubber-stained gantry cranes used to load and unload container ships in major ports. The primary power source in this system is a diesel engine. The supercapacitor, on the other hand, is used together with the diesel engine, easing the diesel engine's workload. In this way, the same work can be done using a smaller-sized diesel engine. This improves air quality while reducing emissions from the diesel engine [8, 40-41].
Supercapacitors are already available on many devices. For example, in cameras, supercapacitors provide power for high power-consuming functions such as zoom mode for close-up shooting and prolonging its life by lightening the load of the battery, which is the main power source [7]. For small portable devices, a supercapacitor can be preferred as an energy storage device with a small volume, lightweight, high reliability, and high mechanical strength [24].
6. Overview of supercapacitor technology
Environmentally friendly power supplies are gaining more and more importance. Energy obtained from sources such as solar energy and wind energy is also clean energy, but it depends on climatic conditions and varies. Therefore, these systems may not always meet the needs. This is one of the reasons why the supercapacitor, which is an environmentally friendly energy storage device, is preferred [38]. Supercapacitors are becoming increasingly popular due to their high specific power and ability to integrate these properties into batteries with high energy density. Hybrid energy storage systems in which battery and supercapacitor are used together are promising. The battery’s high energy density and the supercapacitor's high power density can be exploited simultaneously. This type of system cannot match the performance of conventional internal combustion engines [35]. However, if it is considered that the population in the world is increasing and environmental problems cannot be ignored, the importance of such environmental systems will be understood.
Supercapacitors are profitable systems in the long run in terms of cost. The cost of supercapacitors dropped from 80 cents per farad in 1996 to 10 cents per farad in 2010. It continues to decline [38].
A supercapacitor can operate from temperatures as low as –40 to as high as +85 without loss of performance [7, 42]. For batteries, weather conditions are essential, and they lose performance at shallow temperatures, while at very high temperatures, there is a risk of explosion. This reduces the reliability of the batteries. In order to give an electric vehicle its initial speed and lighten the load on the battery, using an additional supercapacitor on the battery is a useful solution.
The disadvantage of supercapacitors is that they have a low energy density. A supercapacitor can store about 5 % of the energy of a lithium-ion battery. The most important reason is that although batteries store energy in most of their materials, supercapacitors can only store energy on the electrode surface [7]. The larger the electrode surface, the greater the stored energy, but it is not currently possible to store as much energy as a battery. Therefore, hybrid systems are in the foreground instead of a single supercapacitor energizing a device.
As a result, besides the advantages of supercapacitors, such as high power density, fast charge-discharge time, long cycle life, cost-effectiveness, reliability, and durability. It also has disadvantages, such as low energy density. The use of supercapacitors in devices is mostly due to these reasons; a supercapacitor can operate at 85 % to 98 % efficiency, and it can run very fast thanks to its high power density. In addition, the materials from which they are produced are environmentally friendly [15, 36, 40, 43-44].
7. Conclusions
In this article, supercapacitors are examined from various perspectives. In order to better understand supercapacitors, first of all, capacitor technology from the past to the present has been discussed. Afterwards, the structure of the supercapacitor, which is an advanced type of capacitor, its working mechanism and properties such as specific power, specific energy, cycle life, cost, etc. are examined. Electrode materials and electrolyte types, which are of great importance in the structure of the supercapacitor, have been studied in detail. Types of supercapacitors and applications in which supercapacitors are used are other topics in the article.
Supercapacitor technology is a relatively new and emerging technology. A supercapacitor alone cannot currently provide sufficient power to an electrical device. Because supercapacitors have low energy density, it is more advantageous to use them with batteries in electric vehicles. Literature searches have shown that the advantages of supercapacitors and the integration of these advantages into batteries are promising for many applications.
The most important property to be developed in supercapacitors is energy density. The energy density of a supercapacitor is highly dependent on the electrode surface. Many studies have been carried out to increase the electrode surface area. While the electrode surface area of supercapacitors is increasing day by day, their costs are decreasing day by day. In addition to all these, the important point is that supercapacitors are produced entirely from environmentally friendly materials and do not have a negative impact on the environment when they become waste. One of the most important focal points of the 21st century is the concept of “sustainability”. Supercapacitors are one step ahead of other storage devices in this respect. As a result of all this, supercapacitors are promising for applications that require energy storage, both today and in the future.
References
-
A. Kerem, “Elektrikli Araç Teknolojisinin Gelişimi ve Gelecek Beklentileri,” Mehmet Akif Ersoy Üniversitesi Fen Bilimleri Enstitüsü Dergisi, Vol. 5, No. 1, pp. 1–13, 2014.
-
B. Brunekreef and S. T. Holgate, “Air pollution and health,” The Lancet, Vol. 360, No. 9341, pp. 1233–1242, Oct. 2002, https://doi.org/10.1016/s0140-6736(02)11274-8
-
D. Bakker, “Battery electric vehicles, performance, CO2 emissions, lifecycle costs and advanced battery technology development,” Master thesis, Copernicus Institute University of Utrecht, Utrecht, 2010.
-
T. Bocklisch, “Hybrid energy storage approach for renewable energy applications,” Journal of Energy Storage, Vol. 8, pp. 311–319, Nov. 2016, https://doi.org/10.1016/j.est.2016.01.004
-
A. K. Shukla, A. Banerjee, M. K. Ravikumar, and A. Jalajakshi, “Electrochemical capacitors: technical challenges and prognosis for future markets,” Electrochimica Acta, Vol. 84, pp. 165–173, Dec. 2012, https://doi.org/10.1016/j.electacta.2012.03.059
-
A. M. San, “Bir süperkapasitörün elektronik modellemesi,” Maltepe University, Institute of Science and Technology, 2019.
-
J. Schindall, “The charge of the ultracapacitors,” IEEE Spectrum, Vol. 44, No. 11, pp. 42–46, Nov. 2007, https://doi.org/10.1109/mspec.2007.4378458
-
J. R. Miller and A. Burke, “Electrochemical capacitors: challenges and opportunities for real-world applications,” The Electrochemical Society Interface, Vol. 17, No. 1, pp. 53–57, Mar. 2008, https://doi.org/10.1149/2.f08081if
-
P. Sharma and T. S. Bhatti, “A review on electrochemical double-layer capacitors,” Energy Conversion and Management, Vol. 51, No. 12, pp. 2901–2912, Dec. 2010, https://doi.org/10.1016/j.enconman.2010.06.031
-
Y. Zhang et al., “Progress of electrochemical capacitor electrode materials: A review,” International Journal of Hydrogen Energy, Vol. 34, No. 11, pp. 4889–4899, Jun. 2009, https://doi.org/10.1016/j.ijhydene.2009.04.005
-
R. Hemmati and H. Saboori, “Emergence of hybrid energy storage systems in renewable energy and transport applications – A review,” Renewable and Sustainable Energy Reviews, Vol. 65, pp. 11–23, Nov. 2016, https://doi.org/10.1016/j.rser.2016.06.029
-
Kuşdoğan, “Yenilenebilir enerji uygulamalarında hibrit enerji depolama teknolojileri ve uygulamaları,” in 20th International Energy and Environment Fair and Conference, 2017.
-
J. R. Miller and P. Simon, “The chalkboard: fundamentals of electrochemical capacitor design and operation,” The Electrochemical Society Interface, Vol. 17, No. 1, pp. 31–32, Mar. 2008, https://doi.org/10.1149/2.f02081if
-
F. Li, J. Shi, and X. Qin, “Synthesis and supercapacitor characteristics of PANI/CNTs composites,” Chinese Science Bulletin, Vol. 55, No. 11, pp. 1100–1106, Apr. 2010, https://doi.org/10.1007/s11434-009-0573-9
-
I. Hadjipaschalis, A. Poullikkas, and V. Efthimiou, “Overview of current and future energy storage technologies for electric power applications,” Renewable and Sustainable Energy Reviews, Vol. 13, No. 6-7, pp. 1513–1522, Aug. 2009, https://doi.org/10.1016/j.rser.2008.09.028
-
D. Zhang, J. Wang, Q. Wang, S. Huang, H. Feng, and H. Luo, “Nitrogen self-doped porous carbon material derived from metal-organic framework for high-performance super-capacitors,” Journal of Energy Storage, Vol. 25, p. 100904, Oct. 2019, https://doi.org/10.1016/j.est.2019.100904
-
M. Balbaşı and A. Şahin, “Düşük karbon içerikli simetrik süperkapasitör uygulamasi,” Gazi Üniversitesi Mühendislik-Mimarlık Fakültesi Dergisi, Vol. 30, No. 4, pp. 683–692, Dec. 2015, https://doi.org/10.17341/gummfd.63887
-
M. S. W. Chan, K. T. Chau, and C. C. Chan, “Effective charging method for ultracapacitors,” Journal of Asian Electric Vehicles, Vol. 3, No. 2, pp. 771–776, Jan. 2005, https://doi.org/10.4130/jaev.3.771
-
J. Libich, J. Máca, J. Vondrák, O. Čech, and M. Sedlaříková, “Supercapacitors: properties and applications,” Journal of Energy Storage, Vol. 17, pp. 224–227, Jun. 2018, https://doi.org/10.1016/j.est.2018.03.012
-
K. Sharma, A. Arora, and S. K. Tripathi, “Review of supercapacitors: materials and devices,” Journal of Energy Storage, Vol. 21, pp. 801–825, Feb. 2019, https://doi.org/10.1016/j.est.2019.01.010
-
N. I. Jalal, R. I. Ibrahim, and M. K. Oudah, “A review on supercapacitors: types and components,” in Journal of Physics: Conference Series, Vol. 1973, No. 1, p. 012015, Aug. 2021, https://doi.org/10.1088/1742-6596/1973/1/012015
-
R. Kötz and M. Carlen, “Principles and applications of electrochemical capacitors,” Electrochimica Acta, Vol. 45, No. 15-16, pp. 2483–2498, May 2000, https://doi.org/10.1016/s0013-4686(00)00354-6
-
E. B. O. Sihite, Stepanus, and Budiarto, “Study of the effect of supercapasitors types on crystal structure and microstructure of supercapasitor electrode materials,” in IOP Conference Series: Materials Science and Engineering, Vol. 725, p. 012042, Jan. 2020, https://doi.org/10.1088/1757-899x/725/1/012042
-
Y. Wang, X. Wu, Y. Han, and T. Li, “Flexible supercapacitor: overview and outlooks,” Journal of Energy Storage, Vol. 42, p. 103053, Oct. 2021, https://doi.org/10.1016/j.est.2021.103053
-
T. Mehtab et al., “Metal-organic frameworks for energy storage devices: Batteries and supercapacitors,” Journal of Energy Storage, Vol. 21, pp. 632–646, Feb. 2019, https://doi.org/10.1016/j.est.2018.12.025
-
S. Rajagopal, R. Pulapparambil Vallikkattil, M. Mohamed Ibrahim, and D. G. Velev, “Electrode materials for supercapacitors in hybrid electric vehicles: challenges and current progress,” Condensed Matter, Vol. 7, No. 1, Jan. 2022, https://doi.org/https://doi.org/10.3390/condmat7010006
-
Z. Yu, L. Tetard, L. Zhai, and J. Thomas, “Supercapacitor electrode materials: nanostructures from 0 to 3 dimensions,” Energy and Environmental Science, Vol. 8, No. 3, pp. 702–730, Jan. 2015, https://doi.org/10.1039/c4ee03229b
-
E. Frackowiak and F. Béguin, “Carbon materials for the electrochemical storage of energy in capacitors,” Carbon, Vol. 39, No. 6, pp. 937–950, May 2001, https://doi.org/10.1016/s0008-6223(00)00183-4
-
A. Burke, “R&D considerations for the performance and application of electrochemical capacitors,” Electrochimica Acta, Vol. 53, No. 3, pp. 1083–1091, Dec. 2007, https://doi.org/10.1016/j.electacta.2007.01.011
-
Q. Jiang, M. Z. Qu, G. M. Zhou, B. L. Zhang, and Z. L. Yu, “A study of activated carbon nanotubes as electrochemical super capacitors electrode materials,” Materials Letters, Vol. 57, No. 4, pp. 988–991, Dec. 2002, https://doi.org/10.1016/s0167-577x(02)00911-4
-
Y. Ma et al., “A two step approach for making super capacitors from waste wood,” Journal of Cleaner Production, Vol. 279, p. 123786, Jan. 2021, https://doi.org/10.1016/j.jclepro.2020.123786
-
M. N. M. Iqbaldin, I. Khudzir, M. I. M. Azlan, A. G. Zaidi, B. Surani, and Z. Zubri, “Propertıes of coconut shell activated carbon,” Journal of Tropical Forest Science, Vol. 25, No. 4, pp. 497–503, Oct. 2013.
-
E. Taer, R. Taslim, Z. Aini, S. D. Hartati, and W. S. Mustika, “Activated carbon electrode from banana-peel waste for supercapacitor applications,” in 6th International Conference on Theoretical and Applied Physics, Jan. 2017, https://doi.org/10.1063/1.4973093
-
M. Winter and R. J. Brodd, “What are batteries, fuel cells, and supercapacitors?,” Chemical Reviews, Vol. 104, No. 10, pp. 4245–4270, Oct. 2004, https://doi.org/10.1021/cr020730k
-
V. Paladini, T. Donateo, A. de Risi, and D. Laforgia, “Super-capacitors fuel-cell hybrid electric vehicle optimization and control strategy development,” Energy Conversion and Management, Vol. 48, No. 11, pp. 3001–3008, Nov. 2007, https://doi.org/10.1016/j.enconman.2007.07.014
-
A. Caliker and E. Ozdemir, “Modern enerji depolama sistemleri ve kullanım alanları,” in Enerji Verimliliği ve Kalitesi Sempozyumu EVK-2013, pp. 175–179, May 2024.
-
O. Erdinç, M. Uzunoğlu, and B. Vural, “Hibrit alternatif enerji sistemlerinde kullanilan enerji depolama üniteleri,” in Electrical-Electronics and Computer Symposiu, Jan. 2011, https://doi.org/10.13140/2.1.5102.5923
-
S. Satpathy, S. Das, and B. K. Bhattacharyya, “How and where to use super-capacitors effectively, an integration of review of past and new characterization works on super-capacitors,” Journal of Energy Storage, Vol. 27, p. 101044, Feb. 2020, https://doi.org/10.1016/j.est.2019.101044
-
E. Altindemir, “Hibrit elektrikli taşıtlarda rejeneratif frenleme,” Istanbul Technical University Institute of Science and Technology, 2008.
-
D. Cericola, P. Novák, A. Wokaun, and R. Kötz, “Hybridization of electrochemical capacitors and rechargeable batteries: An experimental analysis of the different possible approaches utilizing activated carbon, Li4Ti5O12 and LiMn2O4,” Journal of Power Sources, Vol. 196, No. 23, pp. 10305–10313, Dec. 2011, https://doi.org/10.1016/j.jpowsour.2011.07.032
-
S. Ould Amrouche, D. Rekioua, T. Rekioua, and S. Bacha, “Overview of energy storage in renewable energy systems,” International Journal of Hydrogen Energy, Vol. 41, No. 45, pp. 20914–20927, Dec. 2016, https://doi.org/10.1016/j.ijhydene.2016.06.243
-
B. Kocaman, “Akıllı şebekeler ve mikro şebekelerde enerji depolama teknolojileri,” BEÜ Fen Bilimleri Dergisi, Vol. 2, No. 1, pp. 119–127, Jun. 2013.
-
X. Xu, J. Nan, J. Wang, and Z. Gao, “Estimate of super capacitor’s dynamic capacity,” Energy Procedia, Vol. 105, pp. 2194–2200, May 2017, https://doi.org/10.1016/j.egypro.2017.03.619
-
P. Simon, Y. Gogotsi, and B. Dunn, “Where do batteries end and supercapacitors begin?,” Science, Vol. 343, No. 6176, pp. 1210–1211, Mar. 2014, https://doi.org/10.1126/science.1249625
About this article
The authors have not disclosed any funding.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Saliha Cansu Gorgulu: conceptualization, methodology, resources, visualization, writing-original draft preparation. Isil Yazar: conceptualization, methodology, supervision. Tahir Hikmet Karakoc: supervision.
The authors declare that they have no conflict of interest.
