Abstract
Heart rate variability reflects current functional state of organism, the activity of autonomic nervous system and individual’s adaptive reserves in response to changes in external or internal body functioning conditions. The aim of this study is to evaluate regulatory changes of autonomous cardiac function on systemic level (RR variability), arising during peloidotherapy procedure in persons of different age and gender. The results showed differences in men’s and women’s autonomic nervous system response to peloidotherapy procedure – statistically significant time characteristic changes of HRV in men’s group start to appear only during third stage of the procedure, when humoral mechanisms kick in; in women’s group changes of frequency characteristics and their significant differences starts to appear already at the start of the procedure. Usually autonomic regulation of heart rate of older people is reduced; however heart rate variability changes during peloidotherapy procedure were adequate.
1. Introduction
Autonomic nervous system maintains inner balance - metabolic rate, body temperature, the average breathing rate, arterial blood pressure, heart rate and so on. Increased activity of autonomic nervous system is usually observed during most of chronic diseases. Autonomic nervous system activity by itself does not cause disease, but rather represents a response to actual or expected illness. The impact on autonomic nervous system is one of the most significant actions in balneotherapy and peloidotherapy procedures in treatment of chronic diseases. Autonomic nervous system activity changes during treatment in health resorts – in some cases overall autonomic nervous system tension or just sympathetic activity is reduced. In other cases parasympathetic impulsion is activated or equilibrium between both is restored [1].
Mud procedure acts on the mechanical, thermal, osmotic, chemical and other receptors on the skin and causes complex reactions of human body. Biologically active substances are acting in the skin, and acts through humoral route. Almost all physiological systems are involved into the general reaction of human organism during peloidotherapy. Reaction type and intensity depends on the intensity of mud procedure - temperature of mud, area of application, duration of the procedure, as well as the reactivity of the organism, which is quite difficult to determine [2]. Peloidotherapy is strong thermal and biochemical stimulus that acts primarily on thermoregulatory system which is associated with metabolic rate, cardiovascular system, blood flow redistribution in the body, respiratory function [3].
Heart rate (HR) is a systemic parameter, its changes over time can be easily monitored [4], [5]. Heart rate variability (HRV), as the autonomic nervous system status indicator varies with age [6], is influenced by gender [7]. HRV changes assessed as prognostic indicators of disease for patients with cardiovascular problems - coronary heart disease, heart failure, after acute myocardial infarction [8].
The aim of this study is to evaluate regulatory changes of autonomous cardiac function on systemic level (RR variability), arising during peloidotherapy procedure in persons of different age and gender.
2. Methods
The research was carried out in the health care resort centre “Druskininkų gydykla”.
Procedure – therapeutic peat mud bath (39°C) (peat from “Mašnyčios” peat bog mixed with medium mineralization (~10 g/l) sodium-potassium-calcium chloride mineral water “Sveikata” in 1:2 ratio).
The 12-lead standard ECG was registered synchronously using computerized ECG analysis system “Kaunas-Load”. ECG was registered continuously during whole procedure – 20 minutes. Three segments of equal lengths for 5 minutes were assessed from ECG: the beginning of the procedure 1-5 min (1), the middle of the procedure 7-12 min (2) and the end of the procedure 15-20 min (3). Rhythmograms of each stage were analyzed using computerized ECG registration and analysis system “Kaunas – Load”. Heart rate (HR), RR interval and heart rate variability (HRV) characteristics were analyzed using the standard Fourier transform:
Heart rate variability can be assessed in the time and frequency domains. Measures in the time domain include the standard deviation of the NN interval (SDNN), i.e. the square root of variance. This indicator reflects the parasympathetic effects and is suitable for short RR intervals sequences calculation, because the increase of the sequence length increases other factors influence on heart rate control, as well as the sympathetic influence, especially when the heart rate frequency changes [9]. Other indicator calculated - the square root of the mean squared differences of successive NN intervals (RMSSD), which is used in the assessment of parasympathetic effects during physical exertion or medication taken [9]. It is generally accepted that under resting conditions HRV in the time domain mainly reflects respiratory sinus arrhythmia, which is mediated by parasympatic cardiovagal outflow.
Fig. 1Rhytmogram of women (age 56 years), registered continuously during peloidotherapy
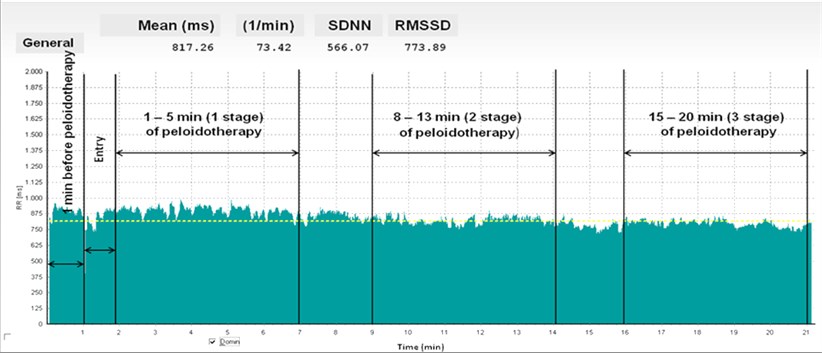
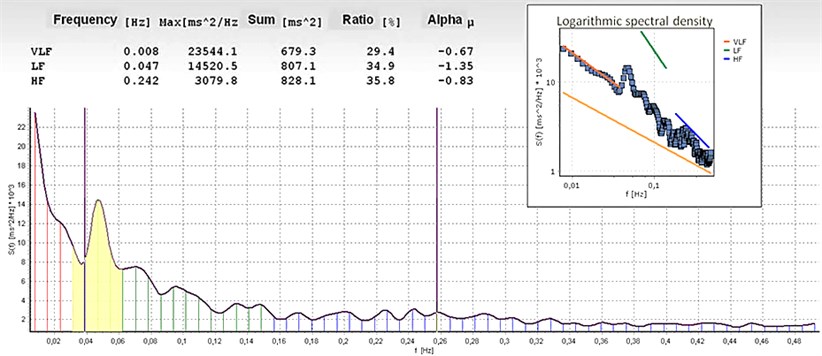
Heart rate range was divided into three frequency bands: very low frequency (VLF2) (from 0.003 till 0.04 Hz), low frequency (LF2) (from 0.04 till 0.15 Hz) and high frequency (HF2) (from 0.15 till 0.4 Hz) components. LF (LF1) and HF (HF1) relative value of each power component in proportion to the total power minus the VLF component has been also calculated. The representation of LF1 and HF1 in n.u. emphasizes the controlled and balanced behaviour of the two branches of the autonomic nervous system [9]. As well, “alpha” – coefficient of direction in log-log spectrum power presentation was calculated, “alpha” shown in tables mean evaluation for all strip in all spectrum, “alpha VLF” – evaluation for VLF of spectrum, “alpha LF” – evaluation for LF and “alpha HF” – evaluation for HF of spectrum. Evaluations of alpha about 0 – white noise, alpha = 1 – pink noise, partially regulated process and alpha = 2 – brown noise, strongly regulated process.
Fig. 2Spectrogram of women (age 56 years), registered continuously during peloidotherapy
Statistical analysis of the data. Data obtained from Fourier analysis was processed using SPSS 17.0. Arithmetic mean and standard deviation of parameters were calculated. The normality of distribution of results was estimated using the Shapiro-Wilk test. The data differed significantly from normal distribution. Reliability of differences of two independent group parameters was determined by the nonparametric Mann-Whitney test for two independent samples. In order to determine the significance of the differences between examinations at the start, in the middle and at the end stages of peloidotherapy procedure, non-parametric Wilcoxon test for paired samples was used. The significance level was chosen at 0.05.
The study was carried out with Kaunas Regional Biomedical Research Ethics Committee permission No. BE-2-50.
3. Results
36 volunteers (16 male and 20 female; age between 30 and 82 years, mean 59.2 ± 11.9) were included in this study. All subjects were with no history of diabetes or cardiac disease.
Peloidotherapy procedures for all subjects were appointed to treat symptoms of musculoskeletal disorders (osteoarthritis, spondylosis and spondyloarthrosis). In order to investigate whether heart rate variability changes during peloidotherapy procedures are influenced by gender, subjects were divided into groups by gender. Group I A consisted of 16 men, average age of 59.125 ± 12.42 and I B group consisted of 20 women with mean age of 59.25 ± 11.74. The two groups did not differ by age. In order to investigate whether heart rate variability is influenced by age, subjects were divided into two groups according to age: II A group consisted of 20 subject’s ≤ 59 years (9 men and 11 women), II B group consisted of 16 subjects aged > 59 years. In this group, there were 7 males and 9 females.
By nonparametric Mann-Whitney test for two independent samples IA and IB groups differed significantly only in the third phase of the variability Coherence (Hz) ( 0.036), and according to age and other indicators of heart rate variability there were no differences. II A and II B groups differed by a second phase of RMSSD ( 0.018), alpha LF ( 0.001), alpha ( 0.049) and the third stage alpha HF ( 0.046). According to other indicators of heart rate variability statistically significant differences were not observed.
Statistically significant changes of parameters throughout the procedure were comparied using non-parametric Wilcoxon test for paired samples.
Table 1Men (I A gr.), HRV changes during peloidotherapy, N= 16. (**** - p< 0.05 during all stages, *** - p< 0.05 between 1 and 2 stage, ** - p< 0.05 between 2 and 3 stage, * - p< 0.05 between 1 and 3 stage, NS – no significant difference)
Time of peloidotherapy | < 0.05 | ||||||
HRV | 1-5 min (1 stage) | 8-13 min (2 stage) | 15-20 min (3 stage) | ||||
Mean | ±SD | Mean | ±SD | Mean | ±SD | ||
Mean of RR (ms) | 851.79 | 120.90 | 806.17 | 103.10 | 772.41 | 85.42 | **** |
1/min | 71.74 | 8.87 | 75.50 | 8.44 | 78.74 | 8.11 | **** |
Time domain | |||||||
SDNN (ms) | 43.29 | 20.87 | 39.38 | 16.19 | 32.18 | 14.45 | **, * |
RMSSD (ms) | 33.72 | 25.79 | 31.63 | 22.45 | 23.72 | 16.43 | **, * |
Freq. domain HRV (without VLF component) | |||||||
HF1 power (%) | 55.60 | 13.55 | 54.85 | 12.18 | 57.60 | 13.07 | NS |
LF1 power (%) | 44.40 | 13.55 | 45.15 | 12.18 | 42.40 | 13.07 | NS |
LF1 + HF1 power (ms2) | 1704.83 | 989.20 | 1678.05 | 963.82 | 1592.71 | 926.44 | NS |
LF1 / HF1 ratio | 0.94 | 0.58 | 0.87 | 0.45 | 0.85 | 0.49 | NS |
Coherence of variability (%) | 40.20 | 8.04 | 39.92 | 10.89 | 42.10 | 11.27 | NS |
Coherence of variability (Hz) | 0.063 | 0.03 | 0.054 | 0.01 | 0.079 | 0.09 | NS |
Freq. domain HRV (with VLF component) | |||||||
VLF2 power (%) | 26.80 | 7.08 | 27.56 | 7.48 | 26.51 | 7.22 | NS |
LF2 power (%) | 32.03 | 9.26 | 31.49 | 8.74 | 30.72 | 9.38 | NS |
HF2 power (%) | 41.18 | 12.44 | 40.95 | 10.52 | 42.75 | 12.43 | NS |
VLF2 power (ms2) | 595.12 | 256.96 | 544.66 | 181.24 | 524.10 | 234.77 | NS |
LF2 power (ms2) | 780.53 | 644.73 | 719.82 | 511.01 | 643.66 | 379.45 | NS |
HF2 power (ms2) | 945.27 | 534.29 | 1020.74 | 764.77 | 950.11 | 696.36 | NS |
Total power (ms2) | 2320.92 | 1184.67 | 2285.22 | 1193.50 | 2117.87 | 1093.23 | NS |
Alpha VLF | –0.54 | 0.15 | –0.44 | 0.32 | –0.46 | 0.31 | NS |
Alpha LF | –1.32 | 0.67 | –1.01 | 0.67 | –1.11 | 0.62 | NS |
Alpha HF | –0.28 | 0.76 | –0.29 | 0.63 | –0.21 | 0.68 | NS |
Alpha | –0.64 | 0.30 | –0.63 | 0.27 | –0.60 | 0.31 | NS |
In evaluating heart rate variability of men during peloidotherapy procedure, we see that standard deviation of the NN interval (SDNN) and square root of the mean squared differences of successive NN intervals (RMSSD) changes at the end of the procedure (15-20 minute of the procedure) and statistically significantly ( 0.05) differs from rates at the first and at the second stages of the procedure. Other evaluated characteristics of heart rate variability have not changed significantly during the procedure.
In Tables 1, 2, 3, 4 we observe statistically significant increase in heart rate and shortening of RR interval throughout the procedure in all groups.
In women group (Table No. 2) we have not founded statistically significant time reading changes of standard deviation of the NN interval (SDNN) and square root of the mean squared differences of successive NN intervals (RMSSD), but statistically ( 0.05) significant heart rate frequency characteristics changes were observed. At the second stage of the procedure (8-13 minute) the relative influence of the sympathetic nervous system and overall power (ms2) of low frequency (LF1) and high frequency (HF1) parameters increases, and remains at the similar levels until the very end of the procedure – there are no statistically significant differences between these indicators at the second and the third stages of the procedure. LF1 / HF1 ratio increases throughout the procedure and statistically significant differences become apparent after 15 minute of the procedure (frequency characteristics without VLF componente).
Table 2Women (I B gr.) HRV changes during peloidotherapy procedures. N= 20. (**** - p< 0.05 between all stages, *** - p< 0.05 between 1 and 2 stage, ** - p< 0.05 between 2 and 3 stage, * - p< 0.05 between 1 and 3 stage, NS – no significant difference)
Time of peloidotherapy | < 0.05 | ||||||
HRV | 1-5 min (1 stage) | 8-13 min (2 stage) | 15-20 min (3 stage) | ||||
Mean | ±SD | Mean | ±SD | Mean | ±SD | ||
Mean RR | 808.10 | 115.42 | 776.62 | 111.43 | 739.04 | 109.77 | **** |
1/min | 74.10 | 13.26 | 79.00 | 11.36 | 83.14 | 11.95 | **** |
Time domain | |||||||
SDNN (ms) | 39.99 | 14.50 | 39.71 | 13.19 | 35.98 | 16.02 | NS |
RMSSD (ms) | 30.57 | 18.07 | 32.14 | 21.39 | 28.18 | 22.08 | NS |
Freq. domain HRV (without VLF component) | |||||||
HF1 power (%) | 60.49 | 9.52 | 57.14 | 8.45 | 55.76 | 8.99 | ***, * |
LF1 power (%) | 39.51 | 9.52 | 42.86 | 8.45 | 44.24 | 8.99 | ***, * |
LF1 + HF1 power (ms2) | 1810.22 | 995.78 | 1553.48 | 918.24 | 1353.95 | 858.11 | ***, * |
LF1 / HF1 ratio | 0.71 | 0.29 | 0.79 | 0.28 | 0.86 | 0.31 | * |
Coherence of variability (%) | 37.40 | 10.02 | 39.81 | 8.90 | 44.84 | 8.32 | **, * |
Coherence of variability (Hz) | 0.107 | 0.12 | 0.054 | 0.02 | 0.067 | 0.11 | **** |
Freq. domain HRV (with VLF component) | |||||||
VLF2 power (%) | 25.92 | 6.34 | 28.80 | 7.40 | 28.89 | 7.72 | ***, * |
LF2 power (%) | 28.85 | 5.86 | 30.02 | 5.18 | 31.08 | 6.09 | NS |
HF2 power (%) | 45.23 | 9.87 | 41.18 | 8.97 | 40.03 | 9.08 | ***, * |
VLF2 power (ms2) | 592.09 | 247.40 | 554.59 | 150.66 | 467.53 | 143.16 | **, * |
LF2 power (ms2) | 697.12 | 337.36 | 652.66 | 351.01 | 590.04 | 364.21 | * |
HF2 power (ms2) | 1139.23 | 685.22 | 927.82 | 576.97 | 763.90 | 522.47 | **** |
Total power (ms2) | 2428.44 | 1102.78 | 2135.07 | 948.16 | 1821.47 | 902.85 | **** |
Alpha VLF | –0.54 | 0.25 | –0.55 | 0.34 | –0.50 | 0.18 | NS |
Alpha LF | –1.07 | 0.76 | –1.32 | 0.91 | –1.10 | 0.62 | NS |
Alpha HF | –0.47 | 0.48 | –0.46 | 0.41 | –0.29 | 0.37 | **, * |
Alpha | –0.57 | 0.23 | –0.63 | 0.21 | –0.64 | 0.23 | *** |
Correlative coherence of variability (%) statistically significantly increases only at the end of the procedure and coherence of variability (Hz) initially statistically significantly decreases and then increases significantly, but does not recover – statistically significant differences were observed during all stages of the procedure.
In evaluating frequency characteristics with VLF componente, overall impact of LF2 component expressed as percentages values contribution to the total variability of HR remain unchanged, and HF2 decreases with the increase in the VLF2 percentage values. During the procedure all parameters of frequency characteristics, that are expressed as absolute values (ms2), changes – total power (ms2), VLF2 power (ms2), LF2 power (ms2), HF2 power (ms2). The only difference is the rate of change (Table No. 2).
The changes in parameters of heart rate variability of younger than 59 years old subjects (Table No. 3), we see, that SDNN decreases during all procedure, but differences start to appear only at the end of the procedure. In evaluating frequency characteristics without VLF componente, at the 8-13 minute (2 stage) of the procedure we observe growing influence of sympathetic nervous system (LF1 power %) and later throughout the procedure the influence of parasympathetic nervous system (HF1 power %) began to grow, so there is no difference between the sympathetic and parasympathetic NS activity at the start and the end of the procedure. Overall power of LF1 and HF1 parameters (ms2) decreases till the middle of the procedure and remains at the same levels till the end of the procedure – there are no statistically significant difference between these values at the second and third stages of the procedure. LF1 / HF1 ratio increases throughout the procedure, but there are no statistically significant differences.
Table 3Younger people (≤ 59 years) (II A gr.) HRV changes during peloidotherapy procedure. N= 20. (**** - p< 0.05 between all stages, *** - p< 0.05 between 1 and 2 stages, ** - p< 0.05 between 2 and 3 stages, * - p< 0.05 between 1 and 3 stages, NS – no significant difference)
Time of peloidotherapy | < 0.05 | ||||||
HRV | 1-5 min (1 stage) | 8-13 min (2 stage) | 15-20 min (3 stage) | ||||
Mean | ±SD | Mean | ±SD | Mean | ±SD | ||
Mean RR | 835.01 | 126.72 | 793.38 | 115.73 | 749.23 | 107.79 | **** |
1/min | 71.72 | 12.46 | 77.17 | 10.32 | 81.79 | 11.16 | **** |
Time domain | |||||||
SDNN (ms) | 40.68 | 19.35 | 36.82 | 14.29 | 33.34 | 14.50 | * |
RMSSD (ms) | 26.12 | 17.10 | 24.50 | 14.58 | 20.86 | 13.86 | NS |
Freq. domain HRV (without VLF component) | |||||||
HF1 power (%) | 56.09 | 11.11 | 53.28 | 10.59 | 54.53 | 10.85 | *** |
LF1 power (%) | 43.96 | 11.11 | 46.72 | 10.59 | 45.47 | 10.85 | *** |
LF1 + HF1 power (ms2) | 1685.47 | 967.55 | 1501.28 | 860.88 | 1287.10 | 593.88 | ***, * |
LF1 / HF1 ratio | 0.88 | 0.46 | 0.92 | 0.42 | 0.93 | 0.43 | NS |
Coherence of variability (%) | 40.00 | 9.04 | 42.01 | 9.20 | 45.52 | 8.73 | **, * |
Coherence of variability (Hz) | 0.11 | 0.12 | 0.053 | 0.01 | 0.045 | 0.01 | **** |
Freq. domain HRV (with VLF component) | |||||||
VLF2 power (%) | 27.66 | 5.69 | 29.62 | 5.38 | 29.59 | 5.65 | NS |
LF2 power (%) | 31.56 | 8.06 | 31.84 | 7.19 | 31.71 | 7.39 | NS |
HF2 power (%) | 40.76 | 9.69 | 38.55 | 8.67 | 38.71 | 9.49 | NS |
VLF2 power (ms2) | 625.61 | 294.23 | 581.16 | 196.35 | 523.22 | 198.66 | **, * |
LF2 power (ms2) | 765.06 | 593.99 | 696.82 | 469.33 | 598.78 | 337.86 | * |
HF2 power (ms2) | 937.17 | 502.55 | 829.02 | 470.68 | 688.32 | 323.56 | **** |
Total power (ms2) | 2327.85 | 1213.05 | 2107.00 | 998.76 | 1810.32 | 756.88 | **** |
Alpha VLF | –0.51 | 0.21 | –0.48 | 0.27 | –0.43 | 0.26 | NS |
Alpha LF | –1.33 | 0.63 | –1.59 | 0.75 | –1.25 | 0.57 | *** |
Alpha HF | –0.49 | 0.72 | –0.47 | 0.62 | –0.40 | 0.58 | NS |
Alpha | –0.67 | 0.23 | –0.70 | 0.21 | –0.69 | 0.25 | NS |
Variability coherence (%) statistically significantly increases at the end of the procedure and differs from first and second stage values. Variability coherence (Hz) statistically significantly decreases throughout the procedure. Components of VLF2, LF2 and HF2 (frequency characteristics with VLF componente) expressed as percentages does not change, but the power of VLF2 range expressed in absolute values decreases at the end of the procedure and differs not only between first and third stage of the procedure, but between second and third stage of the procedure also. Power in LF2 range changes more slowly, differences start to appear only between first and third stage of the procedure. Power in HF2 range and overall power decreases much faster and statistically significant differences can be seen between all stages of the procedure. Alpha LF decreases at the beginning of the procedure and returns to initial values later.
Heart rate variability (SDNN) of older than 59 years (Table No. 4) test subjects during peloidotherapy procedure haven't changed statistically significantly. At the end of the procedure statistically significant increase of variability coherence (%), decrease of power of VLF2 and HF2 range expressed in absolute values (ms2) and faster overall power decrease observed already at the first stage of the procedure. Alpha HF at the end of the procedure increased statistically significantly and differed from indices calculated at the beginning and in the middle stages of the procedure.
Table 4Older people (> 59 years) (II B gr.) HRV changes during peloidotherapy procedure. N= 16. (**** - p< 0.05 between all stages, *** - p< 0.05 between 1 and 2 stages, ** - p< 0.05 between 2 and 3 stages, * - p< 0.05 between 1 and 3 stages, NS – no significant difference)
Time of peloidotherapy | < 0.05 | ||||||
HRV | 1-5 min (1 stage) | 8-13 min (2 stage) | 15-20 min (3 stage) | ||||
Mean | ±SD | Mean | ±SD | Mean | ±SD | ||
Mean RR | 818.16 | 110.05 | 785.22 | 99.36 | 759.67 | 91.85 | **** |
1/min | 74.72 | 10.13 | 77.79 | 10.34 | 80.42 | 9.96 | **** |
Time domain | |||||||
SDNN (ms) | 42.43 | 15.23 | 42.99 | 14.18 | 35.47 | 16.54 | NS |
RMSSD (ms) | 39.29 | 24.73 | 41.18 | 25.48 | 32.88 | 23.88 | NS |
Freq. domain HRV (without VLF component) | |||||||
HF1 power (%) | 61.14 | 11.88 | 59.68 | 8.70 | 59.14 | 10.65 | NS |
LF1 power (%) | 38.93 | 11.94 | 40.33 | 8.70 | 40.86 | 10.65 | NS |
LF1 + HF1 power (ms2) | 1860.77 | 1018.38 | 1743.29 | 1016.21 | 1676.26 | 1135.03 | NS |
LF1 / HF1 ratio | 0.72 | 0.45 | 0.72 | 0.24 | 0.76 | 0.33 | NS |
Coherence of variability (%) | 36.95 | 9.36 | 37.17 | 9.89 | 41.25 | 10.57 | * |
Coherence of variability (Hz) | 0.06 | 0.02 | 0.055 | 0.02 | 0.106 | 0.14 | NS |
Freq. domain HRV (with VLF component) | |||||||
VLF2 power (%) | 24.62 | 7.40 | 26.56 | 9.16 | 25.64 | 9.01 | NS |
LF2 power (%) | 28.64 | 6.92 | 29.21 | 6.49 | 29.93 | 8.01 | NS |
HF2 power (%) | 46.74 | 12.16 | 44.23 | 9.93 | 44.43 | 11.39 | NS |
VLF2 power (ms2) | 553.22 | 175.50 | 511.45 | 99.72 | 454.48 | 173.74 | * |
LF2 power (ms2) | 695.60 | 337.94 | 664.62 | 374.49 | 632.73 | 410.41 | NS |
HF2 power (ms2) | 1197.84 | 736.43 | 1144.24 | 819.85 | 1044.59 | 802.13 | * |
Total power (ms2) | 2446.66 | 1038.50 | 2320.31 | 1134.04 | 2131.80 | 1221.82 | ***, * |
Alpha VLF | –0.57 | 0.21 | –0.53 | 0.41 | –0.54 | 0.21 | NS |
Alpha LF | –1.00 | 0.81 | –0.67 | 0.56 | –0.91 | 0.61 | NS |
Alpha HF | –0.26 | 0.46 | –0.27 | 0.35 | –0.08 | 0.39 | **, * |
Alpha | –0.51 | 0.28 | –0.54 | 0.24 | –0.53 | 0.27 | NS |
4. Discussion of the results
Volume of the procedure, duration, mud temperature and chemical composition was the same for all subjects. During peloidotherapy procedure organism functions in unusual, similar to weightless state, conditions. When lying in a mud bath, a large amount of hot mud is pressing on the surface of the body, mechanically and chemically irritating skin receptors, changing blood circulation and heat exchange conditions [3]. Peloidotherapy causes intense functional changes in the body, and the adaptive organism response depends on the individual body reactivity and adaptive reserves. There are three body reactions to peloidotherapy procedures: the first two – reflexive and neurochemical take place during the procedure, and the third, the impact phase, lasts 2-24 hours after the procedure [2]. During first phase skin receptors are irritated and external energy is transformed into neural excitation pulses that spread to the CNS and cause complex changes in its function. At the second phase biologically active histamine and acetylcholine type substances are formed in the skin, which circulating in blood affect synapses and exposed sites of nerve endings, causing stimulation of all nervous system [2].
Heart rate variability reflects cardiac rhythm management features, autonomic nervous system activity changes and changes of heart rate can be used to assess the functional status of organism and the ability to adapt to changing environmental conditions [11].
Adequate body's regulatory systems reaction, mobilization of central circulatory system and training effect of peloidotherapy procedures represents a moderate increase of sympathetic nervous system activity at the beginning of treatment and the emerging increase of parasympathetic nervous system activity, heart rate variability and exercise tolerance during the treatment. Quantitative criteria of disadaptation is considered to be LF1 > 50 %, HF1 < 30 %, LF1 / HF1 > 2.5 [13].
Studies of many authors shows, that heart rate variability is influenced by subjects gender [7] and age [15]. However we have not found any statistically significant differences between heart rate variability parameters of men and women in separate stages of the procedure, there were only one difference of variability coherence (Hz) in third stage of the procedure. The age groups differed by second stage RMSSD, alpha LF, alpha and third stage alpha HF. However, the study showed different dynamics of heart rate variability of individual groups during peloidotherapy procedure.
Throughout the procedure, all subject groups demonstrated increase of heart rate (HR) and shortening of RR interval duration.
In group I A (men group) (Table No.1) only time characteristic parameters changed. Changes of SDNN which represents overall variability were observed at the third stage of the procedure, i.e. at the end of it. And the decrease of RMSSD value, which represents heart rate variability, shows functional tension of regulatory system and domination of central regulatory mechanisms, and can be seen as an emerging state of compensated fatigue [11].
In women’s group (Table No. 2), statistically significant changes in the all frequency characteristic parameters, except LF2 power (%), could be seen. Speed of dynamics of these parameters also varies – statistically significant differences starts to show at the first half of the procedure – sympathetic influence increases and parasympathetic influence decreases. VLF2 component includes many of periodic oscillations, including thermoregulation and impact of rennin - angiotensin concentrations fluctuations in blood [9], so the relative increase of very low frequency (VLF2) component contribution to the total spectrum reflects the humoral response, and the decreased high frequency (HF2) component levels shows muffled autonomic responses [16]. Increase of LF1 / HF1 ratio confirms increase of activity of sympathetic nervous system. Such a response could be partly assessed as a protective response, manifesting by activation of autonomic regulation circle [11]. Assessing the results, we think, that men have less expressed first (reflexive) peloidotherapy phase, which lasts until mid-procedure, i.e. approximately till 10-13 minute of the procedure, and the reaction of organism is revealed only in third stage of the procedure, with activation of humoral mechanisms. Women, unlike men, have more pronounced and shorter first reflexive phase and earlier starting of the second, neurochemical phase. Differences of investigated parameters become apparent in the first half of the procedure.
Literature analysis of heart rate variability shows that overall variability decreases with age, high frequency oscillations also disappearing, i.e. parasympathetic activity, and responses to physical activity decline and become more complex [9]. Our data of heart energetic spectrum analysis shows, that older people (II B gr.) had 24.6 % humoral and 74.6 % reflective activity at the first stage of the procedure at higher relative parasympathetic (46.7 %) than sympathetic influence (28.6 %) ant it was not statistically significantly different from younger people (II A gr.) cardiac energy spectrum. When assessing the results, we see the increase in relative sympathetic nervous system activity among people less than 59 years age group (Table No. 3) at the start of the peloidotherapy procedure, and then the reaction is stabilizing and parasympathetic influence beginning to grow again. In II B group (Table No. 4) statistically significant changes in these parameters were not observed. During the peloidotherapy procedure in group II B statistically insignificant fluctuations of humoral activity, the relative increase of sympathetic activity and decrease of parasympathetic activity where observed. In group II A (Table No. 3) throughout the procedure the relative increase of humoral activity, relative parasympathetic activity decrease trend, although no statistically significant difference between these parameters were found ( 0.05). Levels of sympathetic activity remain stable. Total power (ms2) and power in HF2 range (ms2) values decreases during all the procedure, and power in very low frequency range (VLF2 power (ms2)) and power in low frequency range (LF2 power ms2)) – only at the end of the procedure. In II B group these values are changing slower (VLF2 power (ms2), HF2 power (ms2), total power (ms2)), or does not change at all (LF2 power (ms2)).
So, we did not found any significant elderly parasympathetic nervous system activity impairment, and heart rate variability parameters showed adequate changes in the nature of the adaptive response in the treatment group to effects of peloidotherapy.
5. Conclusions
Time characteristic parameters changes in group of men and changes of frequency characteristic parameters in women group have shown the differences in adaptive responses for different gender during peloidotherapy procedure. Women’s adaptive response was faster, more pronounced, and is similar to protective body reaction. Autonomic regulation of heart rate is reduced for older people, but the results revealed no significant changes in heart rate variability during peloidotherapy procedure.
References
-
Ponikowska I., Ferson D. Modern Resort Medicine. Warsaw, MediPress, 2009, p. 50-54, (in Polish).
-
Gorinienė G., Gorinaitė A. Physiotherapy and Effects Acting in Resort. Handbook, Kaunas, 2006, p. 124-125, (in Lithuanian).
-
Olefirenko V. T. Water-Heat Treatment. Moscow, 1986, p. 248-249.
-
Stankus A. Nonlinear model of equilibrium dynamics of heart rate. Works of Technology Sciences in West Lithuania, Conference Materials, Klaipėda, Vol. VI, 2008.
-
Buccelletti F., Bocci M. G. et al. Linear and nonlinear heart rate variability indexes in clinical practice. Review Article. Computational and Mathematical Methods in Medicine, Vol. 2012.
-
Wu G. Q., Arzeno N. M. Chaotic signatures of heart rate variability and its power spectrum in health, aging and heart failure. Plos One, Vol. 4, Issue 2, 2009.
-
Beckers F., Ramaekers D., Aubert A. E. Gender-related differences in nonlinear indices of heart rate variability. Memorias II Latino Americano De Ingenieria Biomedica, Habana, 2001.
-
Malik A. M., Bigger J. T., Camm A. J. et al. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation, Vol. 93, Issue 5, 1996, p. 1043-1065.
-
Žemaitytė M.D. Autonomic Regulation of Heart Rate: Mechanism, Interpretation, Clinical Use. Palanga, 1997.
-
Ablonskytė-Dudonienė R., Ereminienė E. Autonomic heart rhythm regulation and its alteration in myocardial infarction and diabetes mellitus. Medicina, Kaunas, Vol. 46(3), 2010, p. 219-230.
-
Baevsky R. M. The methodology. The analysis of heart rate variability: Method of Space Medicine. Copris Handelsgesellschaft MbH Berlin, 1999, http://www.drkucera.eu/pdfs/technologie_de_1.pdf
-
A. Leonaitė, A. Vainoras Heart rate variability during two relaxation techniques in post-MI men. Electronics and Electrical Engineering, Kaunas, Technologija, Issue 5(101), 2010, p. 107-110.
-
Knyazeva T. A., Estenkova M. G. Pelotherapy adequacy to adaptive and reserve abilities in hypertensive patients with concomitant osteoarthrosis. Cardiosomatics, Vol. 2, Issue 3, 2011, p. 76-81.
-
Ramaekers D., Ector H. et al. Heart rate variability and heart rate in healthy volunteers: is the female autonomic nervous system cardioprotective? European Heart Journal, Issue 19, 1998, p. 1334-1341.
-
Ryan S. M., Goldberger A. L. Gender- and age related differences in heart rate dynamics: are women more complex than men? J. Am. Coll. Cardiol., Vol. 24, Issue 7, 1994, p. 1700-1707.
-
Miliauskas P., Žemaitytė D. Diagnostic value of heart rate variability in general anestesia. Medicina, Vol. 38, Issue 2, 2002.
About this article
We are grateful to the Head Doctor of Druskininkai Health Center Virgaudas Taletavičius for his help in preparing this article.
