Abstract
A reduced mechanism of diesel fuel was developed on the basis of Wang’s mechanism. First, for removing the unimportant reactions, an importance index was defined on the basis of computational singular perturbation (CSP). Choosing the importance index of 0.0065, the 140 unimportant reactions were removed, and obtained a smaller mechanism of 403 elementary reactions. Second, 32 global quasi-steady-state (QSS) species were found with the threshold value of 0.005. Finally, the smallest mechanism containing 77-species was obtained. By contrast with the ignition delay time of the original Wang’s mechanism, the maximum error of the final 77-species mechanism was 7.2 %. The final mechanism was also performed with the original Wang’s mechanism and the experiments date from the selected homogeneous charge compression ignition (HCCI) engine, the good overlap of curves was obtained and the maximum error of the simulation results was less than 10 %.
Highlights
- A reduced 77-species diesel fuel mechanism was developed on the basis of Wang‘s mechanism comprising 109 species and 543 elementary reactions.
- The reduction contained two steps. The first step was to remove the 140 unimportant elementary reactions and the second step was to determine the quasi-steady state components.
- Comparing the simulation results of ignition delay time and HCCI combustion, high fidelity of the reduced and original detailed mechanism was described.
1. Introduction
The HCCI engine can be regarded as a compression ignition mode of gasoline engine, which integrates the advantages of gasoline engine and diesel engine, and can fulfill very low NOx and particulate emissions [1-3]. However, the control of the combustion starting point and combustion rate hinders the practical application of HCCI engine. Accurate fuel mechanism is needed to break this barrier, because the HCCI combustion is mainly depended on the chemical kinetics of the fuel mechanism [4], [5].
Diesel fuel is still widely used in internal combustion engines at the present. However, diesel fuel contains hundreds of components, so it is impossible to develop a mechanism for all components. In order to simulate the diesel fuel’s combustion, various surrogates with different representative components were put forward.
The n-heptane was chosen as diesel fuel in the early days, and many mechanisms of n-heptane had been developed [6]-[8]. The drawbacks of single-component surrogate were obvious since only one component was considered. So, the surrogate including a variety of components and even the aromatic components, are being widely used to replace real diesel fuel in combustion simulations. The recently published diesel fuel mechanism (n-heptane and toluene) comprising 109 species and 543 elementary reactions from Wang [9], had been validated by various experiments, such as ignition delay in shock tubes, species concentration distribution in premixed flames and HCCI engine, etc.
The large detailed chemical kinetic mechanisms occupy and use huge CPU time and memory in calculating complex combustion models such as 2D or 3D models in engines. In addition, chemical stiffness and nonlinearity of large chemical kinetic mechanisms even can cause the calculation to terminate. Thus, it is essential to develop mechanism reduction approaches to generate small reduced mechanisms.
The approaches for mechanism reduction are mainly divided into two categories: one is the skeletal reduction, the other is the timescale based reduction. The skeletal reduction deletes the unimportant species and reactions by one or a combination of the following methods: (1) directed relation graph (DRG) [10], (2) DRG with error propagation (DRGEP) [11], (3) path flux analysis (PFA) [12], (4) revised DRG [13], and (5) CSP [14]-[17]. The timescale based reduction identifies the global quasi-steady-state species on the basis of good experience by one or a combination of the following methods: (1) CSP [18], [19], (2) QSS approximation [20], (3) intrinsic low dimensional manifold (ILDM) [21] and other methods [22-24].
In this paper, the unimportant elementary reactions of Wang’s mechanism were first identified and eliminated using an importance index, which could generate a skeletal mechanism with 403 elementary reactions mechanism. Then, the QSS species were identified by applying CSP method, which could generate a final 77 species reduced mechanism. Finally, the final 77 species reduced mechanism was found having high similarity to the original Wang’s mechanism in mimicking ignition delay, species concentration distribution and HCCI combustion characteristic.
2. Reduction strategies
2.1. Skeletal reduction
The importance of reversible reactions that rapidly reach equilibrium was taken as a skeletal reduction criterion, and the formula of the importance index IA,i can be expressed as follows:
The meanings of the parameters i, vA,i and ωi in the formula could be found in reference [8].
Setting a very small number as the threshold value ξ, when IA,i<ξ, the ith elementary reaction will be deemed as unimportant reaction and eliminated.
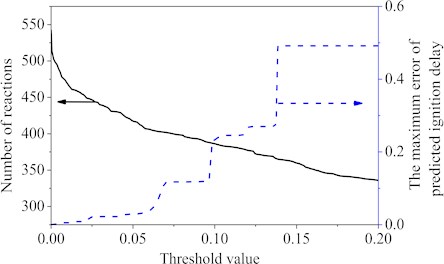
The sampled reduction data came from the auto-ignition results (pressures 10-30 atm, equivalence ratios 0.3-1, temperatures 700 K-1300 K) of the Wang’s mechanism [25]. The c7h8, nc7h16, n2, o2, co2 and h2o of Wang’s mechanism species were picked out as the important species that cannot be removed. The different threshold values were used to remove the unimportant reactions separately. Fig. 1 showed the curves of the reactions number and the maximum ignition delay time error corresponding to the different threshold values. From the Fig. 1, it was easy to see that when the threshold value increased, the maximum ignition delay time error became larger and the elementary reactions were deleted more. Fig. 1 also showed that 140 reactions were subsequently eliminated using a threshold value of approximately 0.0655, and the maximum error of the first reduction was 7 %. Within the set interval, as the threshold value was greater than 0.0655, the maximum error value would be more than 10 %, so the threshold value was chosen as 0.0655 in the first step.
Fig. 1Number of reactions and the maximum error value under different threshold values
2.2. Time scale reduction
The second step was to get quasi-steady-state species, the criterion of getting the quasi-steady-state species can be written in the following form:
where, ε was the threshold value.
Again using the databases from auto-ignition of the Wang’s mechanism, the quasi-steady-state species can be obtained by using the criterion [26]. Fig. 2 showed the curves of the specie number and the maximum ignition delay time error corresponding to the different threshold values. Choosing ε= 0.005, 32 QSS species, namely ic8ketab, tc3h6o2hco, ic3h7co, ac8h16ooh-b, ac8h16ooh-bo2, nc3h7co, ch2cch2oh, ac8h17, nic4h9, ch3co, ac8h17o2, ch2oh, ch, nc7ket24, c7h14ooh2-4, ic4h7o, A1-, tc4h9, tc3h6cho, ic4h5, tc3h6o2cho, ic3h7, c6h5ch2o, nc3h7, c6h4ch3, ch2, pc4h9, hco, c7h15-2, c2h3co and ic3h5co, were obtained. Deleting these QSS species resulted in a final 77-species and 73 global-steps reduced mechanism. From the point of view of ignition delay only, comparing the final mechanism with the original Wang’s mechanism, it was found that the maximum error value of final 77-species mechanism was 7.2 %. Within the set interval, as the threshold value was greater than 0.005, the maximum error value would be more than 10 %, so the threshold value was chosen as 0.005 in the second step.
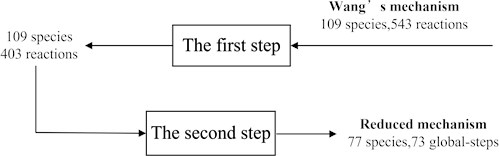
Fig. 3 showed the reduction with two steps. The first step was to remove the 140 unimportant reactions (from 543 reactions to 403 reactions) and the second step was to determine the quasi-steady state components (from 109 species to 77 species). Obviously, the number of species was reduced by one third.
Fig. 2Number of reactions and the maximum error value under different threshold values
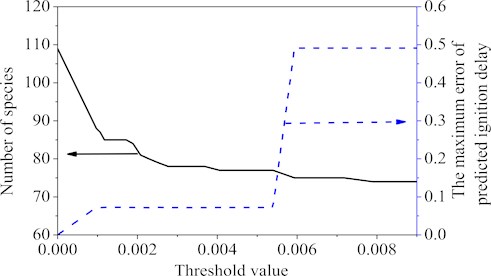
Fig. 3The reduction steps
3. The validation of the final 77-species reduced mechanism
3.1. Ignition delay time
Fig. 4 showed the n-heptane ignition delay curves of Wang’s mechanism and the final 77-species reduced mechanism under the following working conditions: the pressure 40 atm, equivalence ratios 0.5-1 and initial temperatures 700 K-1240 K. No matter high or low temperature, the ignition delay curves of the final reduced mechanism precisely mimicked that of the Wang’s mechanism.
Fig. 4Ignition delay time curves of n-heptane/air mixtures of the Wang’s and reduced mechanisms at various equivalence ratios. Experimental data are obtained from reference [27]
![Ignition delay time curves of n-heptane/air mixtures of the Wang’s and reduced mechanisms at various equivalence ratios. Experimental data are obtained from reference [27]](https://static-01.extrica.com/articles/23132/23132-img4.jpg)
Fig. 5 showed the toluene ignition delay time curves of Wang’s and the final 77-species reduced mechanisms under the following working conditions: the pressure 50 atm, equivalence ratios 0.5-1 and initial temperatures 1000 K-1400 K. It was seen that the very good overlap of the two ignition delay time curves was obtained for the entire parameter range.
Fig. 5Ignition delay time curves of toluene/air mixtures from the Wang’s and reduced mechanisms at various equivalence ratios. Experimental data are obtained from reference [28]
![Ignition delay time curves of toluene/air mixtures from the Wang’s and reduced mechanisms at various equivalence ratios. Experimental data are obtained from reference [28]](https://static-01.extrica.com/articles/23132/23132-img5.jpg)
Fig. 6 showed the ignition delay time curves of two component mixture (n-heptane and toluene) using the Wang’s and the final 77-species reduced mechanisms under the following working conditions: the pressures 10-50 atm, equivalence ratio 0.3 and initial temperatures 800-1250 K. It was seen that the three curves (10 atm, 30 atm and 50 atm) of the final 77-species reduced mechanism were all approximately identical to that of the Wang’s mechanism for the entire parameter range.
Fig. 6Ignition delay time of toluene/n-heptane/air mixtures from the Wang’s and reduced mechanisms at various pressures. Experimental data are obtained from reference [29]
![Ignition delay time of toluene/n-heptane/air mixtures from the Wang’s and reduced mechanisms at various pressures. Experimental data are obtained from reference [29]](https://static-01.extrica.com/articles/23132/23132-img6.jpg)
3.2. Species profiles
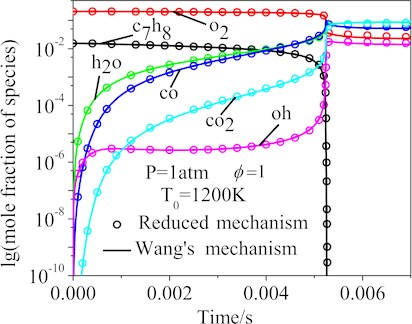
Fig. 7 showed the computed main species mole fraction of the n-heptane/toluene/air mixtures using the Wang’s and the final 77-species reduced mechanisms under the following working conditions: the pressure 1 atm, equivalence ratio 1 and initial temperatures 1200 K. The mole fractions curves of the important species of the Wang’s and the final 77-species reduced mechanisms, such as c7h8, oh, co, o2, co2 and h2o, were in good agreement.
Fig. 7The calculated concentration changes of the main species using the Wang’s and reduced mechanisms
3.3. Validation in HCCI engines
The key parameters of the HCCI engine, such as, compression ratio, bore, stroke, displacement, connecting rod, etc., were from Reference [30].
The simulation conditions were set as follows: 1. the engine speed of 900 r/min, the incipient temperature of 365 K, the incipient pressure of 2 atm and the equivalence ratio of 0.25; 2. the engine speed of 900 r/min, the incipient temperature of 393 K, the incipient pressure of 1atm and the equivalence ratio of 0.2857.
Fig. 8 showed the cylinder pressure curves of the two simulation conditions by using a single zone model. It was easy to see that under given conditions, the cylinder pressure curves calculated by the Wang’s and the final 77-species reduced mechanisms were almost identical (the maximum error of the conditions 1 and 2 was 4.2 %). It was also easy to see that the maximum cylinder pressure calculated by the Wang’s and the final 77-species reduced mechanisms was larger than the experimental data [30]. The deviations were primarily induced by the adiabatic hypothesis.
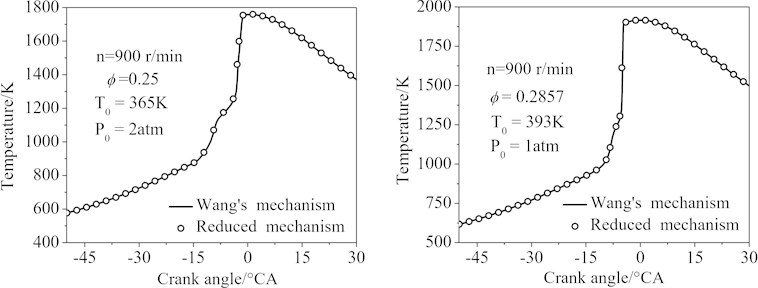
Fig. 9 showed the temperature profiles of the two simulation conditions. It was seen that the curves calculated by the final 77-species reduced mechanism matched that of Wang’s mechanism almost exactly (the maximum error of the conditions 1 and 2 was 6.5 %).
Fig. 8The cylinder pressure of the HCCI engine
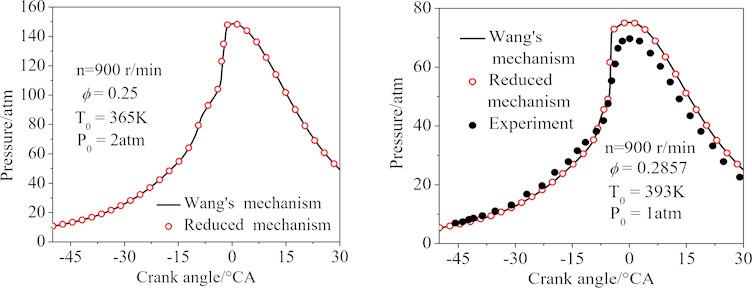
Fig. 9The temperature of the HCCI engine
4. Conclusions
A large diesel fuel mechanism comprising 109 species and 543 elementary reactions was selected as the combustion mechanism of the HCCI engine and reduced to generate a 77 species and 73 global-steps reactions reduced mechanism. The final reduced mechanism was carried out in two steps. The first step was to eliminate 140 unimportant elementary reactions by setting a CSP importance index. In the second step, 32 QSS species were further identified and eliminated. Comparing the simulation results of ignition delay time and HCCI combustion, high fidelity of the reduced and original detailed mechanism was described, and the maximum error of all simulation results was less than 10 %.
References
-
Y. Yamasaki and N. Iida, “Numerical simulation of auto-ignition and combustion of n-butane and air mixtures in a 4 stroke HCCI engine by using elementary reactions,” in CEC/SAE Spring Fuels and Lubricants Meeting and Exposition, Jun. 2000, https://doi.org/10.4271/2000-01-1834
-
T. Aroonsrisopon, V. Sohm, P. Werner, D. E. Foster, T. Morikawa, and M. Iida, “An investigation into the effect of fuel composition on HCCI combustion characteristics,” in SAE Powertrain and Fluid Systems Conference and Exhibition, Oct. 2002, https://doi.org/10.4271/2002-01-2830
-
S. Tanaka, “Two-stage ignition in HCCI combustion and HCCI control by fuels and additives,” Combustion and Flame, Vol. 132, No. 1-2, pp. 219–239, Jan. 2003, https://doi.org/10.1016/s0010-2180(02)00457-1
-
S. Onishi, S. H. Jo, K. Shoda, P. D. Jo, and S. Kato, “Active Thermo-Atmosphere Combustion (ATAC) – A New Combustion Process for Internal Combustion Engines,” in 1979 Automotive Engineering Congress and Exposition, Feb. 1979, https://doi.org/10.4271/790501
-
P. M. Najt and D. E. Foster, “Compression-ignited homogeneous charge combustion,” in SAE International Congress and Exposition, Feb. 1983, https://doi.org/10.4271/830264
-
H. J. Curran, P. Gaffuri, W. J. Pitz, and C. K. Westbrook, “A comprehensive modeling study of n-heptane oxidation,” Combustion and Flame, Vol. 114, No. 1-2, pp. 149–177, Jul. 1998, https://doi.org/10.1016/s0010-2180(97)00282-4
-
H. Curran, “A comprehensive modeling study of ISO-octane oxidation,” Combustion and Flame, Vol. 129, No. 3, pp. 253–280, May 2002, https://doi.org/10.1016/s0010-2180(01)00373-x
-
T. Lu and C. Law, “Strategies for mechanism reduction for large hydrocarbons: N-heptane,” Combustion and Flame, Vol. 154, No. 1-2, pp. 153–163, Jul. 2008, https://doi.org/10.1016/j.combustflame.2007.11.013
-
H. Wang, M. Yao, Z. Yue, M. Jia, and R. D. Reitz, “A reduced toluene reference fuel chemical kinetic mechanism for combustion and polycyclic-aromatic hydrocarbon predictions,” Combustion and Flame, Vol. 162, No. 6, pp. 2390–2404, Jun. 2015, https://doi.org/10.1016/j.combustflame.2015.02.005
-
T. Lu and C. K. Law, “A directed relation graph method for mechanism reduction,” Proceedings of the Combustion Institute, Vol. 30, No. 1, pp. 1333–1341, Jan. 2005, https://doi.org/10.1016/j.proci.2004.08.145
-
P. Pepiotdesjardins and H. Pitsch, “An efficient error-propagation-based reduction method for large chemical kinetic mechanisms,” Combustion and Flame, Vol. 154, No. 1-2, pp. 67–81, Jul. 2008, https://doi.org/10.1016/j.combustflame.2007.10.020
-
W. Sun, Z. Chen, X. Gou, and Y. Ju, “A path flux analysis method for the reduction of detailed chemical kinetic mechanisms,” Combustion and Flame, Vol. 157, No. 7, pp. 1298–1307, Jul. 2010, https://doi.org/10.1016/j.combustflame.2010.03.006
-
Zhaoyu Luo, T. Lu, M. Maciaszek, S. Som, and D. Longman, “A reduced mechanism for high-temperature oxidation of biodiesel surrogates,” Energy and Fuels, Vol. 24, pp. 6283–6293, 2010.
-
S. H. Lam, “Using CSP to understand complex chemical kinetics,” Combustion Science and Technology, Vol. 89, No. 5-6, pp. 375–404, Mar. 1993, https://doi.org/10.1080/00102209308924120
-
M. Valorani, F. Creta, D. A. Goussis, J. C. Lee, and H. N. Najm, “An automatic procedure for the simplification of chemical kinetic mechanisms based on CSP,” Combustion and Flame, Vol. 146, No. 1-2, pp. 29–51, Jul. 2006, https://doi.org/10.1016/j.combustflame.2006.03.011
-
W. Fei and Zhong Beijing, “Skeletal mechanism generation based on eigenvalue analysis method,” Acta Physico-chimica Sinica, Vol. 28, No. 6, pp. 1306–1312, 2012.
-
H. Rabitz, M. Kramer, and D. Dacol, “Sensitivity analysis in chemical kinetics,” Annual Review of Physical Chemistry, Vol. 34, pp. 419–461, 1983.
-
S. Lam and D. Goussis, “The CSP method for simplifying kinetics,” International Journal of Chemical Kinetics, Vol. 26, pp. 461–486, 1994.
-
T. Lu and C. K. Law, “Toward accommodating realistic fuel chemistry in large-scale computations,” Progress in Energy and Combustion Science, Vol. 35, No. 2, pp. 192–215, Apr. 2009, https://doi.org/10.1016/j.pecs.2008.10.002
-
J.-Y. Chen, “A general procedure for constructing reduced reaction mechanisms with given independent relations,” Combustion Science and Technology, Vol. 57, No. 1-3, pp. 89–94, Jan. 1988, https://doi.org/10.1080/00102208808923945
-
U. Maas and S. B. Pope, “Simplifying chemical kinetics: Intrinsic low-dimensional manifolds in composition space,” Combustion and Flame, Vol. 88, No. 3-4, pp. 239–264, Mar. 1992, https://doi.org/10.1016/0010-2180(92)90034-m
-
Y. Chang, M. Jia, P. Wang, B. Niu, and J. Liu, “Construction and derivation of a series of skeletal chemical mechanisms for n-alkanes with uniform and decoupling structure based on reaction rate rules,” Combustion and Flame, Vol. 236, p. 111785, Feb. 2022, https://doi.org/10.1016/j.combustflame.2021.111785
-
B. Niu, M. Jia, Y. Chang, H. Duan, X. Dong, and P. Wang, “Construction of reduced oxidation mechanisms of polyoxymethylene dimethyl ethers (PODE1-6) with consistent structure using decoupling methodology and reaction rate rule,” Combustion and Flame, Vol. 232, p. 111534, Oct. 2021, https://doi.org/10.1016/j.combustflame.2021.111534
-
Y. Chang, M. Jia, S. Huang, H. Zhang, and L. Cai, “Development of skeletal oxidation mechanisms for linear alcohols from C4 to C10 based upon reaction rate rules,” Combustion and Flame, Vol. 245, p. 112351, Nov. 2022, https://doi.org/10.1016/j.combustflame.2022.112351
-
Y. M. Fang, Q. D. Wang, F. Wang, and X. Y. Li, “Reduction of the Detailed Kinetic Mechanism for High-Temperature Combustion of n – Dodecane,” Acta Physico-chimica Sinica, Vol. 28, No. 11, pp. 2536–2542, 2012.
-
Shuhao Li, Jianwen Liu, Rui Li, Fan Wang, Ningxin Tan, and Xiangyuan Li, “Automatic Chemistry Mechanism Reduction on Hydrocarbon Fuel Combustion†,” Chemical Journal of Chinese Universities, Vol. 36, No. 8, p. 1576, Jul. 2015, https://doi.org/10.7503/cjcu20150126
-
M. Hartmann, I. Gushterova, M. Fikri, C. Schulz, R. Schießl, and U. Maas, “Auto-ignition of toluene-doped n-heptane and ISO-octane/air mixtures: High-pressure shock-tube experiments and kinetics modeling,” Combustion and Flame, Vol. 158, No. 1, pp. 172–178, Jan. 2011, https://doi.org/10.1016/j.combustflame.2010.08.005
-
D. F. Davidson, B. M. Gauthier, and R. K. Hanson, “Shock tube ignition measurements of ISO-octane/air and toluene/air at high pressures,” Proceedings of the Combustion Institute, Vol. 30, No. 1, pp. 1175–1182, Jan. 2005, https://doi.org/10.1016/j.proci.2004.08.004
-
J. Herzler et al., “Shock-tube study of the autoignition of n-heptane/toluene/air mixtures at intermediate temperatures and high pressures,” Combustion and Flame, Vol. 149, No. 1-2, pp. 25–31, Apr. 2007, https://doi.org/10.1016/j.combustflame.2006.12.015
-
G. Kalghatgi, P. Risberg, and H.-E. Ångstrom, “A method of defining ignition quality of fuels in HCCI engines,” in 2003 JSAE/SAE International Spring Fuels and Lubricants Meeting, May 2003, https://doi.org/10.4271/2003-01-1816
Cited by
About this article
This work was supported by the Talent Introduction Project of Anhui Science and Technology University (No. RCYJ201902), the Key Projects of Natural Scientific Research in Colleges and Universities of Anhui Province (No. 2022AH051645) and the Transverse Scientific Research Project (Research and Development of Key Technologies of Power Lithium Batteries for Engineering Vehicles).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Chunhui Liu contributed to conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, resources, supervision, validation, visualization and writing – original draft preparation. Delong Zhang contributed to investigation, software and writing – review and editing.
The authors declare that they have no conflict of interest.

