Abstract
New methods and ways of using heterogeneous zeolite catalysts – H-Y, H-Beta, H-MOR, H-ZSM-5 and micro- meso-porous – H-Ymmm and others in organic synthesis were studied. Using the example of available petrochemical compounds such as polyols, olefins, and dienes, the possibilities of carrying out cyclization and condensation reactions in the presence of zeolites of various structural types are shown. It has been established that the H-Beta zeolite catalyst provides a quantitative yield of ethers and esters during the interaction of olefins – substituted vinyl-gem-dichlorocyclopropanes – with linear alcohols and acids, respectively. It has been determined that the condensation of styrene and an equimolar mixture of 4-hydroxymethyl-1,3-dioxolane and 5-hydroxy-1,3-dioxane in the presence of the H-Beta catalyst proceeds with the formation of ethers, while the ratio of 5 and 6-membered isomeric products = 1.2:1. Multicomponent condensations successfully proceed in one stage in the presence of wide-pore zeolites.
1. Introduction
The cyclic acetals and their derivatives are widely use in organic synthesis and find application as a component of solvents, plasticizers, anti-oxidants, inhibitors of the corrosion, bio-effecting agents and etc. [1-3]. Acid-catalyzed condensation of the carbonyl compounds with polyols and cyclization of olefins with formaldehyde (the Prince reaction) are basic manufacturing and preparative directions of creating the cycloacetal structure.
In the literature, there is no generalization and systematization of the results of studying waste-free energy- and resource-saving methods for the synthesis and transformation of cyclic acetals and gem-dichlorocyclopropanes. It was of interest to analyze the available data in terms of their prospects in the preparative synthesis of polyfunctional carbo- and heterocyclic compounds.
This review focuses for the first time on the efficiency of using heterogeneous catalysis methods for the preparation and conversion of cyclic acetals and gem-dichlorocyclopropanes.
Traditional homogeneous catalysts – mineral (phosphoric, sulfuric), organic (vinegar, oxalic) acids, the Lewis acids (ZnCl2, BF3, SnCl4) and other insure high yield and selectivity of targeted heterocycles formation. But their using connects with high consumption of the toxic organic solvents, high energy-wastes with divining and purification of targeted products, control of selectivity process’ difficulty.
We studied the capability of replaced the homogeneous catalysts by zeolites of different modification in this process. This type of perspective heterogeneous catalysts has proven themselves in the isomerization, alkylation, condensation reactions etc. because allows selecting and using the zeolites with required characteristics (size of pores, geometry of channels and acidity) [4-6].
2. Materials and methods
The qualitative and quantitative compositions of the reaction masses were determined using mass spectrometry (using a Chromatek-Kristall 5000M device with the 2012 National Institute of Standards and Technology, USA database) and nuclear magnetic resonance spectroscopy (using a Bruker AM-500 device with operating frequencies of 500 and 125 MHz).
3. Results and discussion
In literature [7, 8] was defined influence of carbonyl compounds structure (formaldehyde, aldehydes, acetone) on total yield of cyclic acetales and ratio of 5 and 6-unit heterocycles’ formation.
It was fond that periodic or continuous condensation of different polyols (ethylene glycol, 1,2 and 1,3-propyleneglycol, glycerin etc.) with carbonyl compounds on acidic zeolites and molecular sieves, which able to absorb aqua, at 120-180°С in 1-4 hours, leads to appropriate 1,3-dioxacycloalkanes with virtually quantitate yield (Fig. 1). While the solvent not required, co-products absent, the stage of neutralization disappear, the catalyst can be use many times without regeneration.
Fig. 1Condensation of ketones and aldehydes with polyols
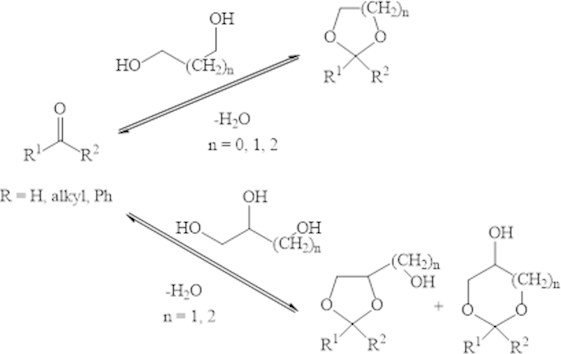
These advantages are saving during the transition to heterogeneous catalytic version of the Prince reaction.
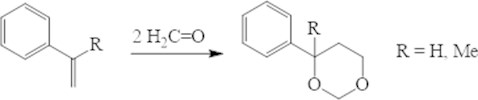
In the presence of the acidic zeolites sterol and α-metylsterol react with the formaldehyde with quantitative formation of the appropriate 4-fenyl-1,3-dioxanes (Fig. 2). The process is ending in 1-2 hours at temperature 80-130 °С.
Fig. 2Condensation of styrene and its analogue with formaldehyde
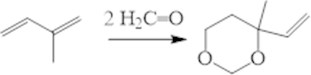
Using of the zeolites in the Prince reaction is more profitable in case of conjugated dienes. From the isoprene with high selectivity formed 4-metyl-4-vinyl-1,3 dioxane, because in conditions of the homogenous catalysis takes place collateral formation of 4-mono and 4,4-disubstituted structures.
Fig. 3Condensation of isoprene and paraformaldehyde
Wide series of gem-dichlorocyclopropanes is approach able based on manufacturing olefins and dienes. This specify the importance of their using in the organic synthesis. So the thermal (400-500°С) isomerization of vinyl-gem- dichlorocyclopropanes is allow obtaining the practically important gem-dichlorocyclopentenes [9], but their yields are less than 25-30 %. We offer the convenientselective obtaining method of the latest (Fig. 4) with heterogeneous-catalytic isomerization of vinyl-gem-dichlorocyclopropanes in presence of the zeolite SAPO-34 [10].
Fig. 4Catalytic isomerization of substituted vinyl-gem-dichlorocyclopropanes
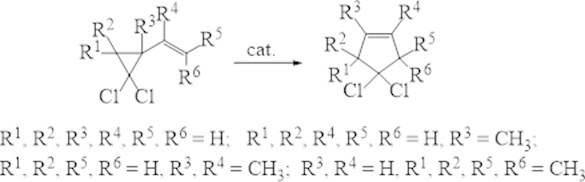
It was found at the 230 °C temperature and continuing of the reaction in 1.5 h yield of targeted cyclopenteneswas ≥ 90 % (selectivity ≥ 95 %).
It was known way from available, based on derivatives of sterol, vinyl-gem-dichlorocyclopropane to derivatives of phenylacroleinin conditions of homogeneous catalysis [11]. It needs to 18-24 h and 5-7-multipleexcess of alcohol for the obtaining of acceptable yields of 60-70 %.
We found [12] that heterogeneous catalyst – anionite АВ-17 allows carry out the reaction of with stoichiometric ratio of the reagents in 4-6 hours at 120-125°С temperature with yield 80-90 % of linear acetals (Fig. 5). The stage of alkaline flow utility and demand of purification and recycling of the solvents are excluded.
Fig. 5Formation of lined acetals in a closed zeolite anionite АВ-17
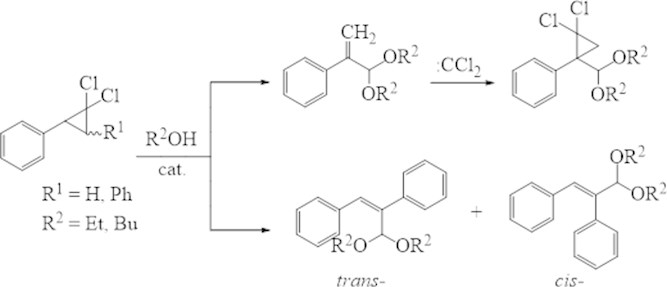
Activity of the 2,3-diphenyl-1,1-dichlorocyclopropane isomers in the decomposition reaction is similar, and they are more active than 2-phenyl-1,1-dichlorocyclopropane. Probably, this is connected with presence of two isomers, which capable of 1,2-migration of benzyl hydrogen’s atoms in 2 and 3 positions.
The structure of synthesized compounds was assigned based by data of NMR 1H, 13C and chromatography-mass spectrometry. The determined signals for the individual cis-, trans-compounds are the protons of the ОCHО group as a kind of singlet with chemical shift 5.77 and 4.94 ppm. respectively. Protons of the ester group CH2O in specter of the trans-acetale resonate at 3.67 ppm., and in cis-isomer – at 3.69 ppm. In 13С NMR specter of trans-acetale the signals of carbonic atoms С = Сappears in more deficient space (114.97-141.16 ppm.) than the signals of similar atoms its cis-isomer (114.45 and 141.12 ppm.). The chemical shifts of carbon in ОСО group have a difference and there are 111.31 and 110.29 ppm. as respectively.
It is known that with homogeneous-catalytic reaction of arenes with vinyl-gem-dichlorocyclopropanes observe the formation o- and n-isomers’ mixture [13]. We studied the reaction of benzole and toluene with the 2-metyl-2-vinyl-gem-dichlorocyclopropane in the presence of different catalysts: microporous - H-Y, H-Beta, H-MOR, H-ZSM-5 and micro-meso-porous-H-Ymmm (Fig. 6).
Fig. 6Alkylation of benzene and its analogues with substituted vinyl-gem-dichlorocyclopropanes
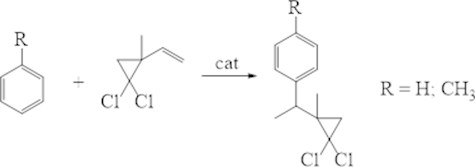
It should be noted that under the studied conditions, the alkylation of toluene with 2-methyl-2-vinyl-gem-dichlorocyclopropane led to the formation of only the p-isomers.
Favorite preparation of ethers and esters by addition of alcohols or mono- and dicarboxylic acids, respectively, to cycloolefin hydrocarbons was carried out in the presence of mineral or organic catalysts [14-20]. Reactions proceed at high temperatures and with low selectivity without a catalyst [21].
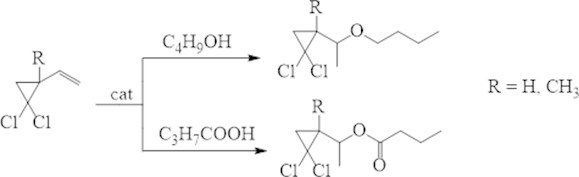
We have described [22, 23] effective methods of ethers and esters preparation in 40-55 % yields by adding butanol or butyric acid to 2-vinyl- and 2-methyl-2-vinyl-gem-dichlorocyclopropanes in the presence of a commercially available zeolite catalyst H-Beta (Fig. 7).
Fig. 7Condensation of substituted vinyl-gem-dichlorocyclopropanes with alcohols and acids
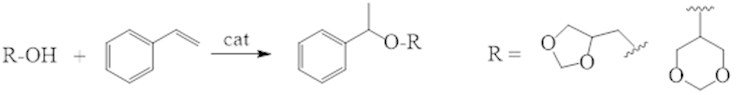
Because esters containing functional groups are interest such intermediates of organic synthesis, we involved in the reaction [22] commercially available styrene and glycerol formals as an equimolar mixture of 4-hydroxymethyl-1,3-dioxolane and 5-hydroxy-1,3-dioxane and styrene. The formation 5- and 6-membered isomers = 1.2: 1 with a yield of 60 % was observed at a temperature 150 °С for 5 hours in the presence of H-Beta catalyst (Fig. 8).
Thus, the transfer of homogeneous catalytic cyclization and condensation reactions to heterogeneous catalysis conditions increases the yield and selectivity of target compounds, simplifies the isolation of aim products, and, as a rule, does not require of expensive toxic solvents using.
Fig. 8Condensation of an equimolar mixture of 4-hydroxymethyl-1,3-dioxolane and 5-hydroxy-1,3-dioxane with styrene
4. Conclusions
Studied new methods and ways of using heterogeneous zeolite catalysts in organic synthesis. Variants of cyclization and condensation are possible in the presence of structural type zeolites. It was found that zeolite catalysts have a number of complex output ethers and esters in the interaction of olefins with alcohols and acids of various structures.
References
-
M. J. Climent, A. Velty, and A. Corma, “Design of a solid catalyst for the synthesis of a molecule with blossom orange scent,” Green Chemistry, Vol. 4, No. 6, pp. 565–569, Dec. 2002, https://doi.org/10.1039/b207506g
-
S. V. Ley, H. W. M. Priepke, and S. L. Warrine, “Cyclohexane-1,2-diacetals(CDA): A New Protecting Group for Vicinal Diols in Carbohydrates,” Angewandte Chemie International Edition in English, Vol. 33, No. 22, pp. 2290–2292, Dec. 1994, https://doi.org/10.1002/anie.199422901
-
A. L. Maximov, A. I. Nekhaev, and D. N. Ramazanov, “Ethers and acetals, promising petrochemicals from renewable sources,” Petroleum Chemistry, Vol. 55, No. 1, pp. 1–21, Jan. 2015, https://doi.org/10.1134/s0965544115010107
-
O. A. Ponomareva, D. L. Chistov, P. A. Kots, and I. I. Ivanova, “Isoprene synthesis from formaldehyde and isobutylene over zeolite catalysts,” Petroleum Chemistry, Vol. 59, No. 7, pp. 711–718, Jul. 2019, https://doi.org/10.1134/s0965544119070156
-
S. P. Bedenko, K. I. Dement’Ev, V. F. Tret’Yakov, and A. L. Maksimov, “The prins reaction over heterogeneous catalysts (a review),” Petroleum Chemistry, Vol. 60, No. 7, pp. 723–730, Jul. 2020, https://doi.org/10.1134/s0965544120070026
-
Isidro M. Pastor and Miguel Yus, “The prins reaction: advances and applications,” Current Organic Chemistry, Vol. 11, No. 10, pp. 925–957, Jul. 2007, https://doi.org/10.2174/138527207781024067
-
J. Deutsch, A. Martin, and H. Lieske, “Investigations on heterogeneously catalysed condensations of glycerol to cyclic acetals,” Journal of Catalysis, Vol. 245, No. 2, pp. 428–435, Jan. 2007, https://doi.org/10.1016/j.jcat.2006.11.006
-
V. R. Ruiz et al., “Gold catalysts and solid catalysts for biomass transformations: Valorization of glycerol and glycerol-water mixtures through formation of cyclic acetals,” Journal of Catalysis, Vol. 271, No. 2, pp. 351–357, May 2010, https://doi.org/10.1016/j.jcat.2010.02.023
-
T. Hudlicky and J.W. Reed, “From discovery to application: 50 years of the vinylcyclopropane-cyclopentene rearrangement and its impact on the synthesis of natural products,” Angewandte Chemie International Edition, Vol. 49, No. 29, pp. 4864–4876, Jul. 2010, https://doi.org/10.1002/anie.200906001
-
S. V. Sokolova, Yu. A. Treger, and O. P. Murasheva, “Obtaining ethylene and propylene from natural gas through the intermediate synthesis of methyl chloride and its subsequent catalytic pyrolysis,” (in Russian), Catalysis in Industry, Vol. 3, p. 15, 2012.
-
S. Kagabu and S. Mizoguchi, “Unique synthetic method for pyridine-ring containing ter-, quater – and quinquearyl and vinylogues by thermolysis of 2,2-dichlorocyclopropylmethyleneamines,” ChemInform, Vol. 372, 1995.
-
G. N. Sakhabutdinova, G. Z. Raskilʼdina, and S. S. Zlotskii, “Synthesis of 2,3-Diphenylacrolein Acetals,” Russian Journal of General Chemistry, Vol. 90, No. 9, pp. 1750–1752, Sep. 2020, https://doi.org/10.1134/s1070363220090248
-
A. R. Khamidullina, E. A. Brusentsova, and S. S. Zlotsky, “Alkylation of benzene and toluene with vinyl-gem-dichlorocyclopropanes,” (in Russian), Chemistry and chemistry Technology, Vol. 51, No. 9, p. 106, 2008.
-
T. T. Dang, F. Boeck, and L. Hintermann, “Hidden brønsted acid catalysis: pathways of accidental or deliberate generation of triflic acid from metal triflates,” The Journal of Organic Chemistry, Vol. 76, No. 22, pp. 9353–9361, Nov. 2011, https://doi.org/10.1021/jo201631x
-
M. K. Mamedov and E. T. Suleimanova, “ChemInform abstract: synthesis of bicyclic esters of monocarboxylic acids,” ChemInform, Vol. 25, No. 18, pp. no–no, Aug. 2010, https://doi.org/10.1002/chin.199418090
-
A. Onopchenko, B. L. Cupples, and A. N. Kresge, “Boron fluoride-catalyzed oligomerization of alkenes: structures, mechanisms, and properties,” Industrial and Engineering Chemistry Product Research and Development, Vol. 22, No. 2, pp. 182–191, Jun. 1983, https://doi.org/10.1021/i300010a006
-
B. Wang, Y. Gu, L. Yang, J. Suo, and O. Kenichi, “Sulfamic acid as a green, efficient, recyclable and reusable catalyst for direct addition of aliphatic acid with cyclic olefins,” Catalysis Letters, Vol. 96, No. 1/2, pp. 71–74, Jul. 2004, https://doi.org/10.1023/b:catl.0000029532.80684.6c
-
A. A. Patwardhan and M. M. Sharma, “Esterification of carboxylic acids with olefins using cation exchange resins as catalysts,” Reactive Polymers, Vol. 13, No. 1-2, pp. 161–176, Sep. 1990, https://doi.org/10.1016/0923-1137(90)90051-5
-
M. A. Harmer and Q. Sun, “Solid acid catalysis using ion-exchange resins,” Applied Catalysis A: General, Vol. 221, No. 1-2, pp. 45–62, Nov. 2001, https://doi.org/10.1016/s0926-860x(01)00794-3
-
A. V. Bogatsky, “Synthesis of 1,4,7-trimethyl-4,5,7,8-tetrahydro-(6H)-imidazo[4,5-e][1,4]-diazepine-5,8-dion-cyclic homolog of caffeine,” (in Russian), Ukrainian journal of Chemistry, Vol. 46, No. 10, pp. 1074–1075, 1985.
-
M. K. Mamedov, E. K. Nabieva, and E. N. Dzhafarova, “Synthesis and conversion of norbornyl esters of acrylic acid,” Russian Journal of Organic Chemistry, Springer Science and Business Media LLC, 2001.
-
G. Z. Raskildina et al., “Heterogeneous catalytic addition of alcohols to terminal olefins,” (in Russian), Bashkir Chemical Journal, Vol. 19, No. 3, pp. 101–105, 2012.
-
G. Z. Raskildina, L. F. Korzhova, N. G. Grigorieva, B. I. Kutepov, A. N. Kazakova, and S. S. Zlotsky, “Heterogeneous catalytic addition of mono-and dicarboxylic acids to olefins,” (in Russian), Chemistry and Chemical. Technology, Vol. 57, No. 1, pp. 36–40, 2014.
About this article
The research was carried out with the financial support of the Priority 2030 program. The authors have not disclosed any funding.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declare that they have no conflict of interest.

