Abstract
In order to understand the reaction characteristics of energetic materials after non-impact ignition, the typical energetic material powder was selected as the research object. The combustion phenomena of ZY-1 and ZY-2 energetic material powder were observed by high-speed photography and thermocouple. The combustion models of two energetic materials were preliminarily constructed through analysis. The arrival time of combustion wave was recorded by thermocouple, and the two kinds of energetic material powders were calculated. The average burning rates of energetic material powders are 4.0 mm·s-1 and 0.49 mm·s-1, respectively.
1. Introduction
On the basis of the heat accumulation of the system itself, additional energy is provided to the system to stimulate or facilitate the rapid response of the system itself, which is called “ignition” [1]. Whether the explosive grows into combustion or detonation after ignition depends on the reaction heat release and the heat and mass transfer effects to the surrounding medium. Explosives are designed for detonation, so explosive researchers usually pay more attention to the detonation performance of explosives, ignoring the combustion performance of explosives, in fact, explosives have a wide range of burning rates [2].
The remarkable sign of the transition from combustion reaction to detonation is the transition from slow “conduction combustion” to violent “convection combustion” [3]. The results show that, in general, the burning rate of explosive decreases with the increase of charge density. This is because with the increase of charge density, the pores of explosive particles are reduced, and the hot gas products are difficult to penetrate into the deep explosive particles, When the charge density is low, the pores between the explosive particles increase, which is conducive to the deep penetration of gas products into the explosive, so as to increase the combustion area and the burning rate [4]. Therefore, it is of great significance for the safety design of explosives to study the density and composition of explosives, as well as the influence of damage on combustion rate [5].
2. Experiment
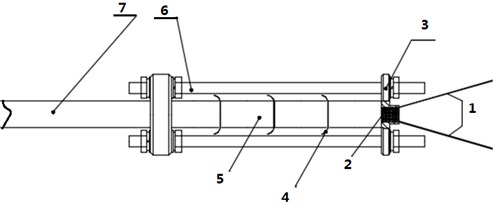
HMX based and RDX based molding powders were selected as typical samples. The equipment used mainly includes measuring cylinder, balance, thermocouple, ignition head, alcohol and absorbent cotton. The test equipment includes high-speed photography: 5000 frames/s; recorder and monitoring equipment. The experimental layout is shown in Fig. 1. The test results mainly come from three aspects: field observation, camera recording and thermocouple data. The burning rate calculation takes the time when the thermocouple temperature reaches 30 °C as the starting point, and uses the piecewise average value to express the flame propagation speed in the sample [6, 7].
Fig. 1A schematic diagram of the experiment layout: 1 – pin wire, 2 – ignition head, 3 – fixed pressing plate, 4 – thermocouple, 5 – sample, 6 – bracket, 7 – strut
3. Results and discussion
ZY-1 and ZY-2 were selected as typical samples for experiments, Calculate the density according to the mass and filling volume of explosive molding powder, the sample parameters and test results are shown in Table 1.
Table 1Two typical samples
No. | Materials | Composition | Density (g·cm-3) |
1 | ZY-1 | HMX/Al/Others | 0.77 |
2 | ZY-2 | RDX/Al/Others | 0.85 |
The change process from ignition to combustion of ZY-1 explosive was photographed by high-speed photography, as shown in Fig. 2. As can be seen from Fig. 2, the flame of ZY-1 is smoothly transferred downward after ignition. Due to the large pressure in the reaction area, high temperature aluminum particles are thrown out, which causes severe combustion when encountering air and bursts out a large number of sparks. The storage length of high-speed photography is only about 1 s, however, it is found that the reaction time is relatively long after the explosive is ignited, so the following experiments use high-definition camera to record the whole process of the experiment.
Fig. 2High-speed photographs of the ignition process of ZY-1 material

a) Before ignition

b) Ignition

c) Combustion flame

d) Severe combustion
Fig. 3Typical flame structure of two energetic materials
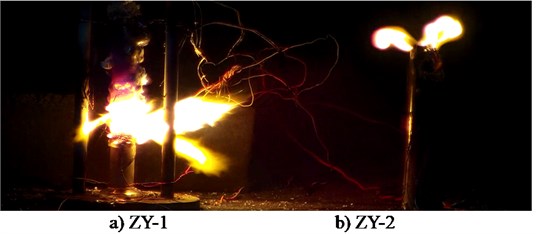
Fig. 3 is the ignition reaction photo of ZY-1 explosive (left) and ZY-2 explosive (right) taken by HD camera, Two samples are ignited at the same time, In 7 minutes and 46 seconds, ZY-1 reacts more than half, However, the reaction process of ZY-2 is difficult to judge directly, In the subsequent processing, the image is partially enlarged and found, There is no flame in the reaction front of ZY-2 explosive, and the flame is always at the upper port. The difference between the two samples proves that the reaction front is not always in plasma state, and the commonly used ionization probe is not suitable for the study of explosive ignition experiments. Fig. 4 is a picture of combustion products of ZY-1 and ZY-2 explosives, and Fig. 5 and Fig. 6 are temperature history curves measured by thermocouples. The experimental results show that although the two kinds of explosives are aluminized explosives, there is a significant difference in the combustion reaction phenomenon. The reaction of ZY-1 explosive is violent, the combustion speed is fast, the reaction of ZY-2 explosive is mild, the combustion speed is slow, and the flame is always located at the upper port of the shell, It shows that the explosive has a rapid decomposition reaction, and the combustible gas burns after encountering air at the upper port. The difference of this phenomenon may be related to the shape of aluminum powder in the explosive, the aluminum oxygen ratio of the explosive, the decomposition temperature of the main explosive and other factors. According to the temperature data in Fig. 5 and Fig. 6, the maximum combustion temperature of ZY-1 explosive is more than 1000 °C, while the combustion temperature of ZY-2 explosive is about 800 °C, and the reaction temperature of the two samples shows a decreasing trend from top to bottom. Through subsequent experiments, it is found that this is almost a common phenomenon, which may be caused by the insufficient reaction due to the decrease of oxygen content in the reaction zone, indicating that the explosive burns under the condition of hypoxia the burning reaction can be inhibited to some extent.
Fig. 4Photograph of the ignition process of two energetic materials

a) ZY-1

b) ZY-2
Fig. 5The temperature history curve of ZY-1 materials
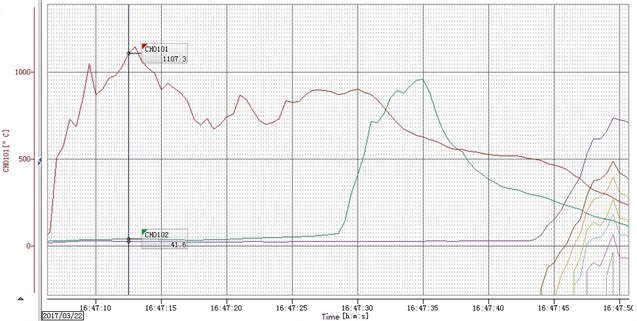
Fig. 6The temperature history curve of ZY-2 materials
According to the above phenomena and thermocouple measurement data, it is judged that the reaction zone structure of ZY-1 and ZY-2 explosives should conform to the model shown in Fig. 7. It is mainly divided into preheating area, decomposition area and combustion area. The main difference is that there is a gas diffusion zone in the reaction of ZY-2 explosive.
Fig. 7Comparison of reaction zone structures of two kinds of materials
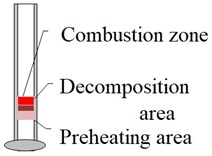
a) ZY-1
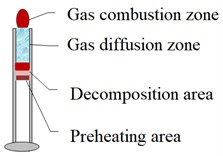
b) ZY-2
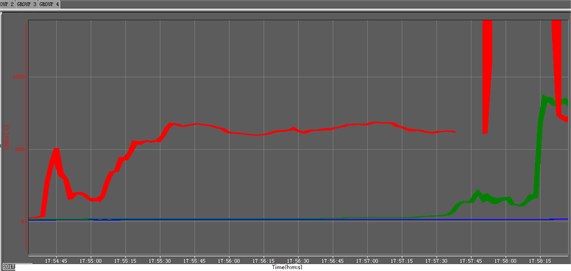
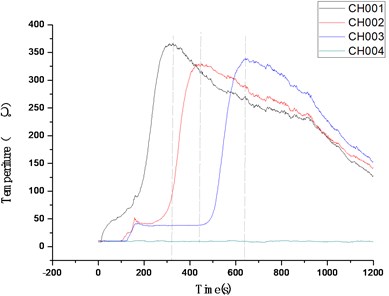
Explosive decomposition is a process in which macromolecules are heated to decompose into micro molecules, At this time, the reaction zone is not in plasma state, and the conventional ionization probe is not suitable for this kind of reaction, Therefore, we choose to use 1 mm diameter thermocouple and HD camera for research, The experimental device is basically the same as that shown in Fig. 1. A fixed support is needed for the explosive with more violent reaction, but not for the explosive with mild reaction. The test results are mainly from three aspects, field observation, camera recording and thermocouple data. A typical experimental curve is shown in Fig. 8. In Fig. 8, the temperature of thermocouple 4 (CH004) has been maintained at the ambient temperature (excluding the possibility of thermocouple abnormality), It can be considered that the reaction of explosives near the bottom of the measuring cylinder stops basically, The average velocity of explosive reaction propagation is calculated by the time interval between three longitudinal dotted lines. The experimental results are shown in Table 2. The average burning rates of the two kinds of explosive molding powder are 4.0 mm·s-1and 0.49 mm·s-1respectively.
Table 2Description of burning speed and burning phenomenon of ZY-1 and ZY-2 materials
No. | Reaction rate / mm·s-1 | Combustion phenomenon | Violence of the reaction |
ZY-1 | 4.0 | Fast combustion, loud noise, large amount of metal combustion sparks | Combustion |
ZY-2 | 0.49 | The combustion is slow and stable, there is no visible flame on the combustion array | Combustion |
ZY-1 is a powdery material containing aluminum, which belongs to the high-temperature volatilization gas phase reaction mode. The basic feature is that the burning rate is relatively large, and the fire is splashing. ZY-2 is a material with a low melting point, the reaction zone is close to the unreacted zone, the combustion reaction rate is slow, and the reaction state is mild. Of course, the main purpose of this article is to explore the combustion characteristics of high-solid energetic materials from a phenomenon perspective. The combustion reaction rate data listed in the article is only a macroscopic relative comparison, not an accurate value.
Fig. 8Typical multiple temperature test curves
4. Conclusions
1) The experimental results show that the reaction of ZY-1 material is violent, the combustion is fast, the reaction of ZY-2 material is mild, the combustion speed is slow, and the flame is always at the upper port. The difference between the two samples shows that the combustion reaction front of the material may not be plasma state, and the ionization probe commonly used is not suitable for the study of material ignition reaction.
2) The average combustion rates of ZY-1 and ZY-2 are 4.0 mm·s-1 and 0.49 mm·s-1, respectively.
References
-
Berghout Laine H., Son Steven F., Hill Larry G., et al. Flame spread through cracks of PBX 9501. Journal of Applied Physics, Vol. 99, 2006, p. 114901.
-
Feng Xiaojun, Tian Xuan, Zhao Juan, et al. Experimental study on the influence factors of DNTF based explosive combustion to detonation. Energetic Materials, Vol. 26, Issue 3, 2018, p. 255-259.
-
Mencius Chun, Zhong Qian, Xu Sen, et al. Study on the influence factors of the minimum self sustaining combustion pressure of emulsion explosive. Blasting Materials, Vol. 47, Issue 2, 2018, p. 12-16.
-
Feng Xiaojun, Zhao Juan, Tian Xuan Experimental study on transition from combustion to detonation of four typical explosives. Journal of Explosives, Vol. 41, Issue 1, 2018, p. 72-76.
-
Zhao Shengwei, Ding Yang, Wang Changli, et al. Experimental study on rapid combustion of Ti black aluminum explosive with shell under rapid heat. Journal of Military Engineering, Vol. 38, Issue 11, 2017, p. 2105-2110.
-
Shang Hailin, Yang Jie, Zhao Feng, et al. Experimental study on ignition and combustion of HMX particle explosive under low velocity impact. Modern Applied Physics, Vol. 8, Issue 2, 2017, p. 67-71.
-
Wu Yanqing, Bao Xiaowei, Wang Mingyang, et al. Ignition combustion mechanism of RDX/HMX particle explosives by drop impact. Explosion and Impact, Vol. 37, Issue 2, 2017, p. 339-346.
